1. De las pociones antiguas a los medicamentos modernos: Un breve viaje a través del uso histórico de los venenos en la medicina
En la historia de la humanidad siempre ha existido un vínculo entre las serpientes y la percepción de la vida y la muerte. De ahí que, los venenos de serpiente hayan sido utilizados de diferentes maneras, desde armas hasta remedios medievales. En algunas lenguas la misma palabra es utilizada para vida y serpiente. Este doble significado explica porque en diferentes culturas las serpientes eran descritas como criaturas sanadoras o como un Dios que da vida [1].
Desde las civilizaciones antiguas, las serpientes han sido vistas como un símbolo de vida y salud. Esto explica porque actualmente las serpientes son el símbolo de la medicina. En las culturas mesoamericanas, Quetzalcóatl (en náhuatl, quetzalli significa «pájaro» y «cóatl» significa serpiente); la serpiente emplumada símbolo de la tierra y el cielo, el tiempo y el espacio, la fertilidad y la creación, además de ser el guardián de la medicina [2]. Actualmente, en la Sierra de Jalisco, México, los curanderos tradicionales de la comunidad Wixárika se mantienen devotos a la serpiente emplumada a través del canto y la danza [2].
En la civilización griega, las serpientes representaban el aire, la tierra y eran ofrendas de Atenea; lo que significaba fecundidad, salud, continuidad, y eternidad. Asimismo, se decía que en los templos de Asklepios en Epidaurus, Rhodes, Cnides, y Cos, el toque de la lengua de una serpiente podía curar la ceguera. También se menciona que Aristóteles logró describir las propiedades y usos de los venenos de las serpientes [3]. Por ejemplo, Aristóteles mencionaba que el veneno de algunas víboras producía síntomas sépticos y muerte inmediata; y que la mordedura de otras denominadas «asp» pudría la carne. Él también pensaba que los animales venenosos obtenían su veneno al comerse otros animales venenosos. Finalmente, Aristóteles describe que las comunidades de Egipto y Libia utilizaban estos venenos para matar otros animales y a sus enemigos [4].
En África, los antiguos egipcios creían que el Ouroboros la serpiente que muerde su cola, representaba el ciclo de la vida, la muerte y la resurrección [5]. Esta civilización antigua se refería a las serpientes como Atoum, la creadora del aire y la tierra. Además, la cobra dorada (Naja haje) llamada Uraeus era el símbolo de la vida, la sabiduría y la soberanía [5]. Se dice que Cleopatra estaba muy interesada en los efectos que causan los venenos de las serpientes [6]. Por lo cual, realizó pruebas en prisioneros condenados para ver las diferentes reacciones corporales y encontrar los límites tóxicos [6].
Adicionalmente, comparó los síntomas de envenenamiento entre especies de víboras (especies de los géneros Cerastes y Echis) y elápidos (especies de los géneros Naja y Walterinnesia). Posteriormente, esta información cumplió otro propósito cuando decidió suicidarse. Al parecer, después de muchos años de estudiar los síntomas y reacciones de numerosos venenos de serpientes en los cuerpos de prisioneros condenados, aprendió que la muerte producida por una víbora era mucho más violenta y dolorosa que la producida por los elápidos. Razón por la cual, eligió al Uraeus real (Naja haje), la cobra de los faraones, y murió a causa de su mordedura silenciosa [6]. Sin embargo, hoy en día la muerte de Cleopatra sigue siendo objeto de controversia. Existen otras teorías, incluidas el asesinato o el suicidio utilizando su conocimiento de plantas venenosas, pero se cree que es altamente probable que su muerte fuera causada por la mordedura de una cobra [6].
Durante el cristianismo y considerando la pésima reputación de las serpientes en la Biblia, es claro por qué en Europa el veneno de serpiente no se usaba frecuentemente. Al respecto se encontraron pocas referencias. Una de ellas es un relato del siglo XIX del químico alemán Kekulé (1829-1896), quien en uno de sus sueños vio una serpiente mordiéndose la cola. Posterior al sueño, relacionó la serpiente del sueño con la estructura de los anillos bencénicos (anillos aromáticos compuestos por seis átomos de carbono utilizados en la preparación de tintes o de detergentes) [3].
La fascinación por la toxicidad de los venenos de serpientes ha llevado a la humanidad a usarlo para matar y curar. Sus usos pueden resumirse en dos categorías: como arma para derrotar silenciosamente a los enemigos; y como tratamientos y remedios para curar a los moribundos. Históricamente, las flechas envenenadas son una de las armas más conocidas. Estas armas tienen su origen en la mitología griega y fueron utilizadas por primera vez por Heracles. Además, desde el sur de África hasta Siberia, muchos soldados y guerreros antiguos usaban flechas recubiertas con veneno de serpientes [3].
En el año 326 a.C., Alejandro Magno fue atacado en la India con uno de estos proyectiles letales. Los síntomas descritos por los soldados sugieren que se trataba del veneno de la víbora de Russell (Daboia russelii). Luego, en el siglo V a.C., los guerreros escitas utilizaron el veneno como recubrimiento de las puntas de las flechas para matar a los enemigos. Sin embargo, esta cultura fue más allá, exploraron las propiedades curativas del veneno. Algunos escritos antiguos describen la técnica empleada por los escitas para obtener los venenos para crear antivenenos y medicinas [7].
El curandero escita Agari se unió al rey Mitrídates (se menciona más adelante) para investigar los venenos de serpiente. Ellos concluyeron que el veneno podía ingerirse en pequeñas cantidades, pero era letal si ingresaba en el torrente sanguíneo [8]. Además, pensaban que toda sustancia venenosa tenía su antídoto natural. Por lo que comúnmente combinaban ingredientes como canela, miel, ricino, ajo, carbón vegetal, con veneno de serpiente para crear diferentes tratamientos. Dentro de los más comunes, se encuentran el Electuary una pasta hecha con miel y una pequeña cantidad de veneno, y el Theriac (=Theriaque), conocido como el primer antiveneno. De este último se creía que estimulaba el sistema inmunológico [9]. Finalmente, el veneno de serpiente también se utilizaba para tratar heridas de guerra y hemorragias, debido a sus propiedades anticoagulantes [10].
El cartaginés Aníbal Barca (247-181 a. C.), general que lideró la lucha contra la República romana durante la Segunda Guerra Púnica, ordenaba a sus soldados arrojar ollas llenas de serpientes a las naves enemigas, causándoles lesiones graves. Sin embargo, este ataque biológico condujo a la creación del primer "antiveneno" al mezclar carne de serpiente con una variedad de componentes y se denominó "Theriaque" [7].
En el imperio del Mar Negro en el siglo I a.C., utilizando los enfoques metodológicos de Cleopatra, el rey Mitrídates VI del Ponto (120–63 a. C.), conocido como el primer toxicólogo, formuló un antiveneno universal que contenía una mezcla de toxinas y antídotos. El rey Mitrídates se autoproclamó el creador del antiveneno (Theriaque) útil en el tratamiento de intoxicaciones producidas por reptiles venenosos y sustancias tóxicas. También usó como método experimental prisioneros para explorar los antivenenos al someterlos a mordeduras de serpientes [11].
Además, se decía que esta sustancia «Theriaque» era capaz de neutralizar todos los venenos de serpientes. Esta técnica asemeja los principios de la inmunización mediante la inoculación de pequeñas cantidades de veneno y, por lo tanto, «preparaba» la respuesta del sistema inmunológico ante la mordedura de una serpiente [12]. Luego, en el año 67 a.C., Mitrídates sufrió una grave herida por el corte de una espada en la pierna, produciéndole un sangrado profuso, dejándolo en riesgo de muerte. Pero uno de sus discípulos logró detener la hemorragia usando veneno de serpiente, salvándole la vida. Este es el primer reporte del uso de venenos de serpiente como coagulante. Este descubrimiento fue utilizado recientemente por científicos en el nuevo campo de la "venómica” [12] (ver Capítulo 5).
Aunque, el “Theriaque” se utilizó ampliamente para múltiples enfermedades por las culturas y civilizaciones del mundo antiguo, fue hasta el siglo XVII cuando el famoso boticario francés, Moyse Charas, publicó la fórmula de Theriaque acabando así con su monopolio. Actualmente, la fórmula original de Theriaque está en desuso. Se puede encontrar en el mercado como un suplemento alimenticio de venta libre que mantiene su nombre, indicando su uso como tratamiento para intoxicaciones alimenticias, para purificar el cuerpo de la brujería, para tratar espasmos digestivos; así como para el trastorno obsesivo-compulsivo y crisis psicológicas [11]. Por favor, no usarlo nunca como antiveneno contra la mordedura de serpientes. Sin embargo, queda bajo su criterio si le es útil en caso de brujería.
Como se puede ver, a lo largo de la historia las guerras antiguas llevaron al desarrollo de varios medicamentos y sustancias curativas que usaban los venenos de serpientes como compuesto activo. Particularmente, se observó un interés casi obsesivo por las serpientes en la literatura médica medieval. Como se mencionó anteriormente, el Theriaque fue uno de los primeros antivenenos. Más tarde, esta sustancia fue producida en París, creándose también una nueva, mucho más simple y cuestionable llamada Orvietan, y otra llamada Bezoard, que contenían hígado y grasa de serpiente, ambas propuestas como la panacea contra los envenenamientos por mordedura de serpiente [3].
Adicionalmente, Lucien Bonaparte en 1843, obtuvo un polvo tóxico llamado Viperine. Al precipitar el veneno de Vipera berus (víbora común europea) con alcohol y éter, comparó sus efectos con los de las enzimas digestivas [13]. Posteriormente, en 1868, el estadounidense Mitchell aisló la toxina crotalina de las serpientes de cascabel y probó sus efectos en animales, obteniendo como resultado principal que producía parálisis [14]. Algunas décadas más tarde, Pelder propuso que los venenos de serpiente tenían un alto contenido proteico. Luego Wolfenden en 1886 validó esta teoría, obteniendo del veneno de serpiente una sustancia similar a la albúmina. Años después de estos descubrimientos, Martin separó el veneno de Pseudechis porphyriacus (serpiente negra de vientre rojo) en dos componentes principales, que, al ser inoculados en animales, uno produce hemorragias, mientras que el otro detiene la respiración [14]. Estos hallazgos representan las primeras descripciones de las actividades biológicas de los venenos (Figura 1).
Otro uso importante del veneno fue la inducción de la respuesta inmune en animales. En 1890, Louis Pasteur conoció al médico francés Albert Calmette (1863–1933), quien fue invitado a crear y dirigir el primer Instituto Pasteur en la Indochina francesa teniendo como centro de operaciones la ciudad de Saigón (ahora de Ho Chi Minh, Vietnam). Su objetivo fue proteger a la población local contra la rabia y la viruela. El Instituto Pasteur abrió en 1891 y Calmette realizó investigaciones sobre los venenos de serpiente. Sus primeros intentos por inducir una respuesta inmune en animales no fueron exitosos [15-16]. No obstante, en 1894 Calmette inoculó a conejos con veneno de cobra, produciendo una respuesta inmune con la que configuró el primer suero anticobra. Calmette presentó sus resultados a la sociedad francesa de biología en el mismo año [17]. Finalmente, en el Instituto Pasteur en Francia, Calmette inició la producción de suero anticobra para uso terapéutico mediante la inyección de veneno de serpiente en caballos, siguiendo lo sugerido por Sewall para lograr una producción masiva [18].
En América Latina, el pionero en la producción de antivenenos fue Vital Brazil, quien leyó el manuscrito publicado por Calmette y se empeñó en replicar sus resultados con serpientes brasileras [5]. Un año después de la publicación de Calmette, Brazil ingresó al Instituto de Bacteriología y comenzó inmediatamente a inocular animales con pequeñas dosis de venenos de serpientes brasileñas [18,19]. En 1901, Vital Brazil publicó sus hallazgos sobre los venenos de serpiente y dio inicio a la producción de suero [20,21]. En febrero de 1901, el Instituto Butantan fue inaugurado oficialmente bajo el nombre de Instituto de Sueroterapia con Vital Brazil como director [6].
Durante el siglo XX, se aislaron y conocieron los efectos fisiológicos y las composiciones de los venenos de diferentes especies de serpientes. Estos se clasificaron por sus propiedades. Por ejemplo, los venenos de Naja naja (cobra común), Crotalus adamanteus (serpiente cascabel de diamantes) y Daboia russellii (víbora de Russel) tienen efectos depresores y coagulantes. Además, se añadió a los diccionarios farmacológicos un nuevo término ofiotoxina, el cual hace referencia a las sustancias presentes en los venenos de serpientes [3].
Posteriormente, se realizaron algunos experimentos que demostraron que los venenos de Crotalus adamanteus, Agkistrodon piscivorus (boca de algodón) y Naja atra (cobra china) podrían afectar el sistema nervioso, produciendo parálisis respiratoria. Asimismo, se validó que existen venenos con propiedades coagulantes como los de los vipéridos Daboia russelii, Echis carinatus (forsa), Trimeresurus gramineus (víbora del bambú) y Crotalus adamanteus; y los elápidos Pseudechis porphyriacus y Acanthophis antarcticus (víbora común de la muerte) [3].
En la segunda mitad del siglo XX, Sergio Ferreira reportó que una fracción del veneno de Bothrops jararaca (jararaca) podía inhibir la conversión de angiotensina I a II, potenciando la acción de la bradicinina (ver detalle más adelante). Esta fracción se denominó factor potenciador de bradiquinina (BPF) [22]. Simultáneamente, Ferreira se unió al laboratorio de John Vane; y Vane se convirtió en consultor de la farmacéutica Squibb. En paralelo, pero de forma independiente, Ferreira et al. [23], y Ondetti et al. [24] otro investigador de Squibb, continuaron investigando para obtener información más precisa sobre BPF, lo que condujo al aislamiento y caracterización de los péptidos activos de la fracción [23,24].
Estos péptidos no tenían suficiente potencial para ser un producto farmacéutico ya que carecían de estabilidad oral y su producción masiva era costosa. Además, Cushman et al. [25] publicaron la caracterización de algunos análogos de los péptidos originales, así como de la región específica que se une al sitio activo de la enzima convertidora de angiotensina I (ACE-I), bloqueando la conversión de angiotensina II y provocando vasodilatación. Posteriormente, Squibb invirtió millones de dólares para generar una forma oralmente activa del fármaco, así como en estudios clínicos y en animales. Finalmente, a principios de la década de 1980, la agencia estadounidense que aprueba las licencias de producción y venta de medicamentos (Food and Drug Administration, FDA) aprobó el Captopril® como fármaco para el tratamiento de la hipertensión. Esto permitió dar tratamiento y evitar las secuelas de la hipertensión arterial como lo son el daño renal, infarto, encefalopatía, entre otros [26].
Entre los años 1980 a 2000, se utilizaron dos desintegrinas derivadas de veneno de serpiente como compuestos principales para el desarrollo de dos fármacos antitrombóticos (Eptiftibatide, Integrilin®, Millenium Pharmaceuticals) y Tirofiban (Agrastat®, Merck & Co) [27]. Actualmente, las toxinas del veneno de serpiente han demostrado múltiples actividades biológicas, como lo son la acción antitumoral, antibacteriana, antiviral, hipotensora, anticoagulante, entre otras [28–30]. Asimismo, los venenos de serpientes se han empleado como herramientas en la investigación biomédica. Por ejemplo, algunas toxinas han sido esenciales para describir cómo funcionan los receptores de membrana celular o el modo de acción de las enzimas. Asimismo, las proteínas del veneno de serpiente son empleadas como reactivos esenciales en la química clínica [28,31].
Por lo tanto, disciplinas como la bioquímica, la fisiología, la farmacología y la inmunología aportan nuevos conocimientos sobre estas toxinas, particularmente sobre su estructura, efectos y reacciones sobre los sistemas orgánicos. Este conocimiento permite la síntesis de principios activos y fármacos mucho más selectivos y efectivos. Desde esta perspectiva, los venenos de serpientes son una fuente esencial de componentes moleculares que pueden modular varios procesos fisiológicos. Hoy en día, existe una variedad de principios activos derivados de los venenos de serpientes. Estos se pueden clasificar por sus efectos, como anticancerígenos, anticoagulantes, manejo del dolor, antibióticos, antihipertensivos, entre otros (Figura 2). Este capítulo aborda el asombroso potencial terapéutico y el uso biomédico de las toxinas del veneno de serpiente.
Figura 1. Infografía de línea de tiempo que muestra los principales usos del veneno de serpiente a lo largo de la humanidad.
2. Rol farmacológico de los venenos de serpiente
El conocimiento sobre las propiedades farmacológicas de los venenos de serpiente ha surgido con el avance de la ciencia y la tecnología. Actualmente, se pueden encontrar gran variedad de principios activos derivados de estas sustancias. Los venenos de serpiente son una mezcla de sustancias activas, entre ellos enzimas, proteínas y péptidos (ver Capítulo 5). Los principales componentes de los venenos incluyen alrededor de 19 familias de proteínas, como las fosfolipasas A2, proteasas de serina, metaloproteinasas, lectinas, L-aminoácido oxidasas, factores potenciadores de bradicinina, factores natriuréticos y antagonistas de integrinas, entre otros [32]. Además, estas sustancias tienen actividades biológicas con potencial terapéutico al interactuar con receptores endógenos (p. ej., receptores de membrana celular acoplados a proteína G y canales iónicos, entre otros), proteínas de membrana y factores de coagulación; afectando el sistema neuromuscular, cardiovascular, la coagulación sanguínea, así como causando alteraciones importantes en el organismo [33].
Adicionalmente, la especificidad, afinidad y selectividad de sus toxinas, ofrecen una excelente oportunidad para nuevas intervenciones farmacológicas, con mecanismos de acción más efectivos, menores interacciones negativas y efectos adversos. Estas características son esenciales en la elaboración del «fármaco ideal», el cual podría producirse a partir del veneno de serpiente [34]. Actualmente, existen numerosos nuevos fármacos para uso humano derivado de sus toxinas con efectos antihipertensivos, antitrombóticos, anticoagulantes, antivirales, analgésicos, neuromoduladores y fibrinolíticos (Tabla 1). Aunque estas toxinas son útiles como fármacos, también pueden ser útiles en el diagnóstico y estudios de mecanismos celulares [35].
Las propiedades terapéuticas atribuidas a los venenos se pueden agrupar en los siguientes usos farmacológicos: primero, alteraciones del sistema cardiovascular y del sistema nervioso; y otras indicaciones, como el tratamiento del cáncer, la terapia antimicrobiana, el manejo de los trastornos de la coagulación, etc. Estas propiedades terapéuticas se abordarán en las siguientes secciones (Figura 2).
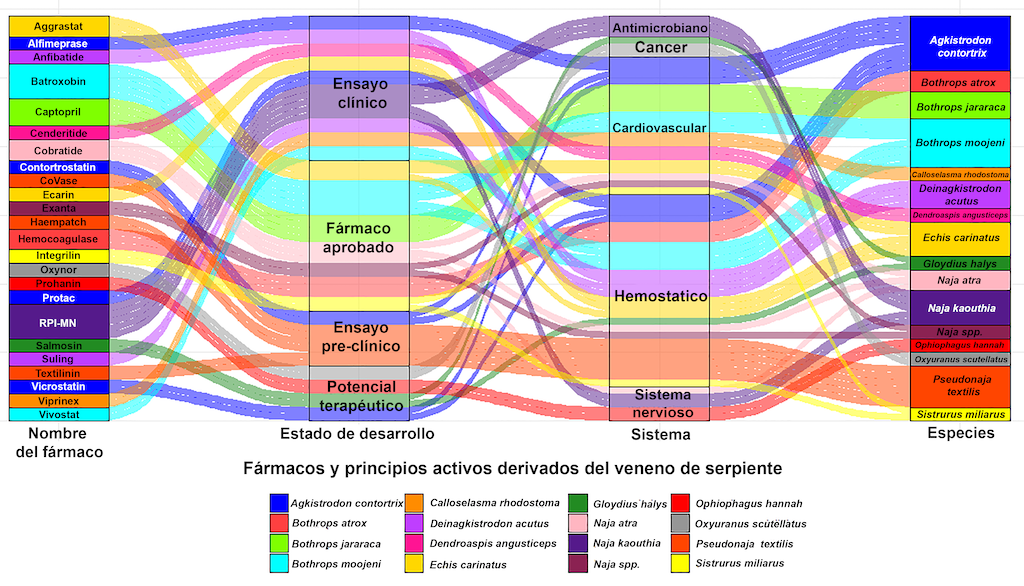
Figura 2. Diagrama aluvial que representa la relación entre los fármacos o compuestos activos, el veneno de serpiente, los sistemas humanos y el estado de desarrollo del farmacológico.
Tabla 1. Actividades biológicas de algunas toxinas del veneno de serpiente y su potencial farmacológico.
|
Toxina
|
Actividad biológica
|
Potencial
|
Referencias
|
|
PLA2
|
Miotoxicidad, neurotoxicidad, modulación de la agregación plaquetaria, anticoagulación, formación de edema e hipotensión
|
Actividad citotóxica, antiviral y antibacterial
|
[36–40]
|
|
SVMPs
|
Hemorragia, miotoxicidad, formación de edema, formación de ampolla y dermonecrosis
|
Activación de los factores de coagulación V y X
|
[41–43]
|
|
SVSPs
|
Alteraciones hemostáticas e hipotensión
|
Hidrólisis de los coágulos sanguíneos, accidente cerebrovascular isquémico y reactivos en química clínica
|
[44–46]
|
|
LAAOs
|
Hemorragia, formación de edema y modulación de la agregación plaquetaria
|
Actividad antibacterial, antileishmania, antiviral y citotoxicidad
|
[47,48]
|
|
Desintegrinas
|
Inhibición de la agregación plaquetaria
|
Agentes anticáncer y antitrombóticos
|
[27,49]
|
|
Lectinas
|
Alteraciones hemostásicas y respuesta inflamatoria
|
Agentes anticáncer y antitrombóticos
|
[46,50]
|
|
3FTxs
|
Neurotoxicidad, cardiotoxicidad, desórdenes hemostáticos e interacción con los canales iónicos
|
Actividad analgésica y agentes útiles en el estudio de la estructura y función de receptores muscarínicos (receptores parasimpáticos)
|
[51–53]
|
Para conocer el mecanismo de acción y el potencial biológico de estas toxinas, consulte el Capítulo 5 de este libro.
Sistema cardiovascular
Las alteraciones del sistema cardiovascular son unas de las enfermedades más frecuentes a nivel mundial. Estas incluyen presión arterial alta, enfermedades isquémicas cardíacas y enfermedades cerebrovasculares. Por ejemplo, entre las causas frecuentes de muertes naturales en hombres y mujeres en Colombia las cardiopatías isquémicas ocupan el 36,9 %, seguidas por enfermedades cerebrovasculares (14,7 %), e hipertensión (8,2 %) [54].
Considerando la gran variabilidad de la etiología y los mecanismos involucrados en la aparición de estas patologías, su manejo representa un desafío para los sistemas de salud. Algunas de estas enfermedades están asociadas a lesiones en los vasos sanguíneos, dislipidemias, insuficiencia cardíaca, alteraciones del ritmo y frecuencia cardíaca, y aterosclerosis, etc. [55]. Además, la aparición de estas patologías puede estar ligada a factores de riesgo genéticos o externos, así como a los hábitos de cada persona. Esto significa que el manejo de estas patologías requiere una amplia gama de medicamentos específicos para tratar la condición particular que dio lugar a la patología [55].
La hipertensión arterial sistémica es una condición crónica caracterizada por una alteración vascular que provoca presión arterial anormalmente alta. Esta enfermedad conduce a eventos vasculares aterotrombóticos (p. ej., infarto agudo de miocardio, evento cerebrovascular, etc.), insuficiencia cardíaca o insuficiencia renal. Sin embargo, en la mayoría de los casos no se logra identificar la causa y requiere un tratamiento crónico que incluye diferentes mecanismos [56,57].
En general, el abordaje para el tratamiento de la hipertensión involucra cambios en el tono vascular y el volumen sanguíneo. Esto se puede lograr inhibiendo o bloqueando los efectos de las sustancias vasoconstrictoras (p. ej., adrenalina, noradrenalina, vasopresina, angiotensina II, aldosterona, hormona antidiurética) o potenciando la acción de los agentes vasodilatadores (p. ej., óxido nítrico, histamina, bradicinina, sustancia P y péptido intestinal vasoactivo) [58]. Para modificar el volumen sanguíneo se utilizan medicamentos diuréticos. Dependiendo del fármaco seleccionado, estos medicamentos producen eliminación de agua y algunos electrolitos (sodio, potasio, magnesio, calcio, entre otros) [58].
Péptidos potenciadores de bradicinina (BPP)
Como se mencionó anteriormente, el Captopril® fue el primer fármaco aprobado por la FDA derivado del péptido potenciador de bradicinina del veneno de serpiente (Bothrops jararaca; Tabla 2). Desde entonces se han sintetizado una gran variedad de fármacos que pertenecen al grupo de los inhibidores de la enzima convertidora de angiotensina [26]. Los péptidos potenciadores de bradicinina están presentes en venenos de vipéridos y elápidos de géneros como Agkistrodon, Bitis, Bothrops, Crotalus, Lachesis, Hydrophis,Oxyuranus, y Pseudonaja [59].
El péptido potenciador de bradiquinina actúa como inhibidor de la enzima convertidora de angiotensina (ECA), siendo uno de los grupos de moléculas más utilizados para tratar la hipertensión ya que produce la relajación de los vasos sanguíneos y una disminución del volumen sanguíneo [60]. Además, previenen la conversión de angiotensina I a angiotensina II, impidiendo su efecto vasoconstrictor, así como la retención de sodio y agua. Esta inhibición también potencia los efectos vasodilatadores y antifibróticos de la bradicinina, que se acumula cuando se inhibe la ACE. Este efecto es especialmente importante en pacientes con enfermedad renal [60]. Asimismo, se han observado efectos citoprotectores de fármacos derivados de BPP en el músculo cardíaco, los cuales podrían ser beneficiosos en insuficiencia cardíaca e infarto agudo de miocardio [60].
Los beneficios del BPP no se limitan a actuar como inhibidores de la ECA. Estos péptidos promueven la síntesis de óxido nítrico (agente vasodilatador endógeno). Además, la BPP puede unirse a los receptores muscarínicos M1 provocando vasodilatación al activar la respuesta del sistema parasimpático [61]. Esto conduce a una disminución sostenida de la presión arterial y a una posible nueva molécula para tratar la hipertensión [26].
Adicionalmente, BPP afecta el sistema nervioso central y modifica la liberación de neurotransmisores como GABA y glutamato. Estos efectos neurológicos podrían modular funciones autonómicas como la presión arterial, la frecuencia cardíaca y la liberación de neurotransmisores. Aunque se necesitan más estudios, estos hallazgos ofrecen nuevas oportunidades para tratar la hipertensión y otras afecciones autonómicas [26].
Gouda et al. [62] plantean la hipótesis de que un fármaco derivado de estos BPP podría ser eficaz para el tratamiento de la COVID-19, dado el rol de la ECA en el mecanismo de replicación del virus. La evidencia sugiere que el virus podría alterar la función de la ECA, afectando la producción de bradicinina. Por lo tanto, inhibir la ECA con BPP de los venenos de serpiente podría resultar en un fármaco eficaz para tratar la COVID-19. Aunque esta hipótesis requiere más evidencia, los procesos fisiológicos demuestran plausibilidad biológica [62].
Péptidos natriuréticos
Los péptidos natriuréticos (péptido natriurético auricular, péptido natriurético cerebral, y péptido natriurético tipo C) son agentes vasoactivos que provocan la alteración de la permeabilidad vascular, aumento de la capacitancia venosa (la capacidad de las venas para almacenar sangre), inhibición del sistema renina-angiotensina-aldosterona (encargado de regular la retención y eliminación de sodio y agua, tono vascular, etc.), diuresis y natriuresis. En otras palabras, los péptidos natriuréticos tienen un rol importante en la regulación del tono vascular, la retención o eliminación de agua y sodio, mediante la modificación de diferentes mecanismos [61].
Recientemente, se han encontrado péptidos natriuréticos en el veneno de la serpiente Dendroaspis angusticeps (mamba verde). Este péptido provoca vasodilatación estimulando la guanilato ciclasa y los canales de calcio en los miocitos aórticos, lo que provoca la relajación del músculo liso vascular [46,61]. Asimismo, se han aislado péptidos natriuréticos del veneno de Crotalus oreganus (serpiente de cascabel del Pacífico Norte), mostrando un efecto vasodilatador al aumentar la producción de óxido nítrico al estimular los canales de potasio. Estos péptidos también se pueden encontrar en el veneno de especies diversas y no relacionadas, como Bungarus flaviceps (krait de cabeza roja), Bungarus multicinctus (krait bandeada), Crotalus durissus (cascabel suramericana), Lachesis muta (verrugoso amazónico), Micrurus corallinus (coral pintada), Oxyuranus microlepidotus (taipán de tierra adentro), Pseudonaja textilis (serpiente marrón oriental), Pseudechis australis (serpiente mulga), Pseudocerastes persicus (víbora persa cornuda),y Protobothrops flavoviridis (serpiente habu) [46,59].
A pesar de las diferentes fuentes de veneno de serpiente, los péptidos natriuréticos tienen los mismos efectos vasodilatadores, lo que permite estudiar y sintetizar esta molécula utilizando diferentes especies. Los medicamentos derivados de este péptido se pueden utilizar para tratar patologías del sistema cardiovascular dado que se ha demostrado un efecto vasodilatador y citoprotector sobre las células cardíacas. Estas propiedades benefician a los pacientes con insuficiencia cardíaca congestiva y cardiopatía isquémica [46,61,63].
Bloqueadores de los canales de calcio
El calcio juega un papel esencial en la contracción muscular. Cuando este ión se libera a través de los canales de calcio, la actina y la miosina interactúan para producir la contracción muscular. Aunque existen diferentes tipos de canales de calcio (por ejemplo, T, L, N, P, Q, R), algunas moléculas del veneno de serpiente como las proteínas 3FTx y PLA2 actúan específicamente en los canales de tipo L y T [40]. Por otro lado, las funciones cardiovasculares del canal de calcio tipo L incluyen la contracción del músculo liso y la función de marcapasos del músculo cardíaco. Los canales de calcio tipo T están implicados en el potencial de acción de los miocitos cardíacos [38]. Al bloquear los canales de calcio (tipo L), se impide el transporte de calcio en el músculo cardíaco y en el músculo liso vascular, lo que provoca vasodilatación, disminución de la frecuencia cardíaca y contractibilidad cardíaca. Esto es especialmente útil para controlar la presión arterial alta, la angina de pecho, las arritmias y la insuficiencia cardíaca [64].
Considerando la importancia de que los medicamentos sean altamente selectivos, las toxinas del veneno de serpiente representan una gran oportunidad para desarrollar medicamentos altamente selectivos dirigidos a los canales de calcio. Este desarrollo evitaría los efectos adversos de los fármacos actualmente disponibles en el mercado, los cuales presentan una muy baja selectividad frente a múltiples alteraciones cardiovasculares y neurológicas. Por ejemplo, las proteínas 3FTx como la calciseptina, toxina FS2, C10S2C2 y S4C8 derivadas de venenos de serpientes elápidas, han mostrado una alta selectividad al unirse y bloquear los canales de calcio de tipo L.
Además, los principios activos sintetizados a partir de estas toxinas pueden utilizarse para tratar otras enfermedades del sistema cardiovascular mencionadas previamente. Por lo tanto, estas toxinas tienen un alto potencial de sustituir a fármacos como el amlodipino o el verapamilo (bloqueantes de los canales de calcio), entre otros; evitando efectos adversos como las palpitaciones, hipotensión, edemas en pies y tobillos, fatiga, vértigo, cefaleas, somnolencia, náuseas, dispepsia y boca seca [63].
Receptores adrenérgicos
Los receptores adrenérgicos son receptores de membrana acoplados a proteínas G responsables de las respuestas simpáticas a la adrenalina. Esto significa que tienen un papel clave en la regulación de la frecuencia cardíaca, la contracción cardíaca, tono vascular, lipólisis, liberación de insulina, broncodilatación, función renal, entre otros. Estos receptores se dividen en α y β con 9 subtipos (seis para α1A, α1B, y α1D; α2A, α2B, α2C; y tres para β1, β2 y β3). Los receptores adrenérgicos están presentes en varios tejidos e intervienen en diferentes respuestas simpáticas fisiológicas [65].
En los tejidos cardiovasculares, los receptores α1, α2 y β2 están presentes principalmente en las células del músculo liso de los vasos sanguíneos [65]. De igual manera, los α1 y β1 también se encuentran en el músculo cardíaco. La activación de los receptores β conduce al aumento de la frecuencia cardíaca, así como al incremento de la contractilidad y velocidad de conducción cardíaca. Los receptores α1 también aumentan la contractilidad cardiaca, provocando vasoconstricción, mientras que los β2 producen relajación del músculo liso. Como resultado, medicamentos como los bloqueadores beta (p. ej., propranolol, carvedilol, metoprolol, entre otros) en combinación con otros tratamientos, se usan ampliamente en patologías como angina de pecho, insuficiencia cardíaca congestiva, hipertensión, arritmias cardíacas, infarto de miocardio, taquicardia, enfermedad cardíaca coronaria, migraña, ansiedad y temblor esencial [65].
Por lo tanto, algunas β-cardiotoxinas (3FTx) del veneno de Ophiophagus hannah (cobra real) se han caracterizado por la unión o bloqueo de los receptores β1 y β2 provocando disminución de la frecuencia cardíaca, mostrando su utilidad en enfermedades cardiovasculares como insuficiencia cardíaca, alteraciones en el ritmo y cardiopatías isquémicas; pero careciendo de los efectos hemolíticos y líticos observados en los fármacos utilizados actualmente. Asimismo, las toxinas AdTx1 y UniProtKB P85092 (3FTx de elápidos) son bloqueadores de los receptores α1 y podrían utilizarse como vasodilatadores [35,46]. Adicionalmente, toxinas como UniProtKB C0HJR1, UniProtKB y C0HJR2 (3FTx) presentes en el veneno de Micrurus mipartitus (rabo de ají) han demostrado modular el neurotransmisor GABA, reduciendo la presión arterial y la frecuencia cardíaca mediante un mecanismo central [63].
Finalmente, algunas cardiotoxinas como las que se encuentran en el veneno de Ophiophagus hannah aumentan la frecuencia cardíaca. Se sabe que estas sustancias causan perturbaciones en la membrana celular y tienen factores líticos. También se denominan citotoxinas y se han estudiado para el tratamiento del cáncer, así como tener algunas propiedades antimicrobianas (esto se abordará más adelante) [35].
Sistema nervioso
Algunos venenos de serpientes contienen componentes neurotóxicos que interrumpen la comunicación entre el axón y la placa motora, produciendo inmovilización y pérdida de coordinación en la presa. Además, estos agentes neurotóxicos actúan sobre mecanismos fisiológicos que son importantes en la neurotransmisión, como los receptores de canales iónicos en las membranas celulares [34,66]. Efectos fatales como parálisis muscular se pueden encontrar tanto en Viperidae (especies de cascabeles del género Crotalus) como en Elapidae (serpientes marinas: Hydrophiinae; especies de corales del género Micrurus). Los venenos de las serpientes elápidas son principalmente ricos en PLA2 y 3FTx, potentes neurotoxinas que interfieren en la transmisión neuromuscular a nivel presináptico o postsináptico [32,67].
Neurotoxinas presinápticas
Los efectos presinápticos se atribuyen principalmente a las β-neurotoxinas. Estas actúan a través de la inhibición de la liberación de acetilcolina al eliminar sus vesículas, lo que resulta en un bloqueo de la transmisión neuromuscular sin interferir con la sensibilidad de la placa motora a la acetilcolina (Ach) [66,68]. Esta característica es útil, ya que un fármaco sintetizado a partir de estas toxinas podría ser un buen candidato para reemplazar los bloqueadores neuromusculares disponibles actualmente (p. ej., despolarizantes: succinilcolina; no despolarizante: rocuronio, vecuronio, atracurio, cisatracurio, mivacurio). Asimismo, estos nuevos fármacos podrían evitar los efectos secundarios habituales como la inestabilidad hemodinámica (taquicardia, bradicardia, hipertensión o hipotensión); broncoespasmo, hipertermia maligna, hiperpotasemia, alteración de la transmisión neuromuscular, lesiones musculares y sus interacciones [67,69].
Neurotoxinas postsinápticas
Por otro lado, las α-neurotoxinas tienen actividad postsináptica, bloqueando de forma reversible los receptores colinérgicos. También, estas toxinas se unen a los receptores nicotínicos de acetilcolina postsinápticos, impidiendo la apertura de los canales iónicos e interrumpiendo la transmisión neuromuscular en la placa motora [66,68]. Este mecanismo ha sido útil para dilucidar la fisiopatología de algunas enfermedades neuromusculares como la miastenia gravis que causa debilidad en los músculos esqueléticos, la cual empeora después de períodos de actividad y mejora después de períodos de descanso [68].
Toxinas analgésicas
Otro uso de la interacción entre estas neurotoxinas y las vías colinérgicas es un efecto beneficioso antinociceptivo, útil en el tratamiento del dolor crónico. Por ejemplo, la toxina de cobra aislada del veneno de Naja naja (cobra común) tiene propiedades analgésicas. Esta toxina es un ligando específico para el receptor colinérgico a1 nAChRα1, que causa efectos analgésicos por una vía no dependiente de opioides [35,53]. Asimismo, la toxina de cobra se une con alta afinidad al nAChRa7, produciendo el mismo efecto. Se ha reportado que esta toxina podría reemplazar a la morfina, ayudando a controlar el síndrome de abstinencia que causa este fármaco [70]. Actualmente, se está estudiando la posible actividad analgésica de la α-neurotoxina de la Ophiophagus hannah (cobra real). De igual manera, la crotamina de Crotalus durissus (serpiente cascabel suramericana) ha demostrado un efecto antinociceptivo más potente que el de la morfina [33,67,71].
Por otra parte, las neurotoxinas (dendrotoxina) del veneno de Dendroaspis angusticeps (mamba verde) se unen selectivamente a los receptores muscarínicos de acetilcolina. Los receptores muscarínicos tienen un papel fundamental en el tratamiento de enfermedades neurodegenerativas como el Alzheimer y el Parkinson. El bloqueo selectivo de estos receptores podría ayudar a restablecer el movimiento normal en estas enfermedades [71,72]. Asimismo, la toxina (Glu-Val-Trp) del veneno de Bothrops atrox (mapaná) ha mostrado propiedades neuroprotectoras y pro-neuroplásticas. En particular, los cultivos de células dopaminérgicas tratadas con esta toxina han mostrado una disminución significativa de la viabilidad celular. Este efecto tiene el potencial de tratar la degeneración neuronal patológica, siendo potencialmente útil en las terapias utilizadas para las enfermedades de Alzheimer y Parkinson [73].
Tabla 2. Fármacos o principios activos aprobados por la FDA para su uso en humanos.
|
Medicamento
|
Mecanismo
|
Fase
|
DP
|
Sistema
|
Indicación
|
Origen
|
Referencia
|
|
Captopril
|
Inhibidor de la enzima convertidora de angiotensina (IECA)
|
Aprobado por FDA
|
Si
|
Cardiovascular
|
Hipertensión, insuficiencia cardíaca congestiva, infarto de miocardio y neuropatía diabética
|
Bothrops jararaca
|
[29,32,35]
|
|
Aggrastat
(Tirofiban)
|
Inhibidor de la glicoproteína IIb/IIIa
|
Aprobado por FDA
|
Si
|
Cardiovascular, hemostásico
|
Infarto de miocardio, Enfermedad coronaria aguda, y terapia antitrombótica
|
Echis carinatus
|
[29,61,71]
|
|
Integrilin
(Eptifibatide)
|
Inhibidor de la glicoproteína IIb/IIIa
|
Aprobado por FDA
|
Si
|
Cardiovascular, hemostásico
|
Enfermedad coronaria aguda y terapia antitrombótica
|
Sistrurus miliarus
|
[29,33,35]
|
|
Defibrase/Reptilase (Batroxobin)
|
Convierte el fibrinógeno en fibrina
|
Aprobado por FDA
|
No
|
Cardiovascular, hemostásico
|
Evento cerebrovascular, tromboembolismo pulmonar, trombosis venosa profunda e infarto de miocardio
|
Bothrops atroxy B. moojeni
|
[32,33,71]
|
|
Hemocoagulase
|
Cataliza los coágulos sanguíneos
|
Aprobado por FDA
|
No
|
Hemostásico
|
Cirugía plástica, cirugía abdominal, y vitrectomía humana
|
Bothrops atrox
|
[33,35,74]
|
|
Exanta (Ximelagatran)
|
Inhibidor directo de trombina
|
Aprobado por FDA
|
No
|
Cardiovascular, hemostásico
|
Complicaciones tromboembólicas de la fibrilación auricular
|
Pool de venenos de Cobra (Naja spp)
|
[29,32,74]
|
|
Cobratide (Ketongning, cobratoxin)
|
Bloqueo de los receptores nicotínicos
|
No está aprobado por la FDA, pero está disponible para su uso en China
|
No
|
Cardiovascular, hemostásico, Sistema nervioso
|
Ciática crónica, artralgia crónica, cefalea neuropática
|
Naja kaouthia Naja atra
|
[29,32,71]
|
DP: Disponible en Colombia
Cáncer
El cáncer es una de las enfermedades más comunes en el mundo. En Colombia, según el Ministerio de Salud y Protección Social, para el año 2020 la incidencia fue de 182 casos por cada 100.000 habitantes. La detección temprana y tratamiento adecuado reducen dramáticamente la mortalidad, por tal razón, para los sistemas de salud la medicina preventiva y la creación de nuevos tratamientos son una prioridad [75].
Por definición, el cáncer es un desorden celular, en cual las células se dividen de forma anormal, formando lesiones que crecen deteriorando los tejidos circundantes, utilizando los recursos energéticos del cuerpo y afectando negativamente la fisiología [76]. Este proceso se denomina carcinogénesis [76]. Además, las células cancerosas pueden migrar desde su tejido original invadiendo otros sistemas, provocando un crecimiento anormal en cualquier sitio. Este proceso se conoce como metástasis [77]. El crecimiento desbordado de estos tejidos anormales produce alteración que con el paso del tiempo compromete el funcionamiento normal de los sistemas del organismo, provocando complicaciones y la muerte en algunos casos [78].
En general, el crecimiento desbordado de las células se debe a una alteración genética, como resultado de la interacción entre los factores genéticos del paciente y agentes externos, tales como: carcinógenos físicos como la radiación ultravioleta e ionizante; carcinógenos químicos como el asbesto, componentes del humo del tabaco, aflatoxinas (contaminantes en los alimentos) y arsénico (contaminantes en el agua potable); y carcinógenos biológicos como virus, bacterias y parásitos [79]. El daño genético o las mutaciones provocadas por estos factores permiten que las células se dividan a mayor velocidad, generando clones con la misma mutación o daño. Posteriormente, las células «hijas» acumulan diversas mutaciones que les permiten crear diferentes tipos de clones. Estas tienen una mayor capacidad de crecimiento y proliferación, en comparación con las células sanas [80].
En condiciones normales, el sistema inmunológico puede controlar las células tumorales mediante un proceso denominado inmunovigilancia tumoral. Sin embargo, algunos clones tienen la capacidad de evadir los mecanismos de control, causando neoplasia (aumento en el número de nuevas células) [81]. También, algunas alteraciones en el sistema inmunológico pueden provocar desequilibrios entre los procesos de proliferación y reproducción celular, así como entre los mecanismos de control del crecimiento y la apoptosis (mecanismo programado de muerte celular) [82].
Por lo tanto, los procesos celulares involucrados en el cáncer son la señalización de proliferación sostenida, la evasión de supresores de crecimiento, la evasión de destrucción inmune, la evasión de la apoptosis, y la consecución de inmortalidad replicativa.
Asimismo, en estados avanzados de la enfermedad, se produce la inducción de angiogénesis (nuevos vasos sanguíneos que alimentan el tumor), invasión y metástasis, mutación e inestabilidad genómica, inflamación promovida por el tumor, y la desregulación de la energía celular [83]. De ahí que el tratamiento del cáncer se centre en interrumpir los procesos de replicación celular, por medio de mecanismos como la alteración del entorno de la célula cancerosa, activación de procesos de muerte celular (apoptosis, autofagia, necrosis), o señalización para alertar al sistema inmunológico de la presencia del tumor [84,85].
Aunque la patología por sí sola causa daño significativo en el cuerpo, los tratamientos como la quimioterapia, la radioterapia e inmunoterapia, en la mayoría de los casos, también lesionan varios sistemas del cuerpo [86]. Adicionalmente, estas intervenciones médicas inhiben el crecimiento o causan la muerte de células sanas, produciendo efectos secundarios importantes (pérdida de cabello, uñas débiles, sangrado, fallas en otros sistemas como el riñón, el hígado, etc.). Dada la dificultad de producir fármacos más selectivos, actualmente existe un gran interés en encontrar fármacos específicos para tratar cada tipo de cáncer conocido [86].
Actualmente, se han creado fármacos más seguros para pacientes con cáncer al mejorar su selectividad y especificidad. Como se mencionó anteriormente, las toxinas del veneno de serpiente son candidatas prometedoras para lograr este objetivo. Numerosas investigaciones han demostrado que estas toxinas pueden provocar un efecto lítico directo sobre las células tumorales o alterar el entorno creado por el tumor para su supervivencia (p. ej., angiogénesis). Además, algunas toxinas desencadenan una respuesta inflamatoria que ayuda a alertar al sistema inmunológico sobre la presencia del tumor [87–90]. Varios estudios han demostrado que las toxinas del veneno de serpiente tienen una eficacia específica contra diferentes tipos de cáncer, como el cáncer de cuello uterino, cáncer de mama, cáncer de páncreas y cáncer de ovario [91–95]. A continuación, describimos brevemente la acción anticancerígena conocida de las toxinas del veneno de serpiente.
Fosfolipasa A 2 (PLA 2)
Entre las toxinas presentes en los venenos de víboras sudamericanas como Bothrops asper, B. jararacussu, B. pauloensis y B. moojeni se ha encontrado que las toxinas PLA2 son útiles para combatir el cáncer [84,87,89]. En particular, la actividad antitumoral de PLA2 está dada por su citotoxicidad, su capacidad de hidrólisis de fosfolípidos y movilización de ácido araquidónico, lo que resulta en la inhibición del crecimiento tumoral, daño en el ADN, apoptosis, autofagia y supresión de metástasis (Tabla 3). Además, estas propiedades interfieren con la proliferación vascular, afectando la angiogénesis tumoral [88,96].
La crotoxina (un tipo de PLA2) del veneno de la serpiente de cascabel sudamericana (Crotalus durissus)ha mostrado actividad citotóxica específica contra las células cancerígenas [87,96]. Esta toxina interfiere con el receptor del factor de crecimiento epidérmico que es altamente selectivo. En algunos ensayos de fase I y II, la crotoxina mostró una reducción de la enfermedad en diferentes tipos de cáncer, como el cáncer de mama, melanoma y leucemia [88–90,95].
Cardiotoxina-3
Como se describió anteriormente, la cardiotoxina presente en el veneno de algunos elápidos tiene propiedades citotóxicas útiles en el tratamiento del cáncer. Esta induce la muerte celular apoptótica y regula los procesos de proliferación [89]. Adicionalmente, algunos estudios han reportado que la cardiotoxina activa la apoptosis en el retículo endoplásmico y en la vía mitocondrial, siendo eficaz como fármaco antineoplásico [88]. Actualmente, la molécula VRCTC-310-Onco, compuesta por crotoxina de Crotalus durissus y cardiotoxina Naja atra, está siendo probada en cáncer de piel y de mama, demostrando una reducción del tumor hasta un 80% de los casos [88,95,96].
Desintegrinas
Las desintegrinas (proteínas no enzimáticas, p. ej., Salmosin) de la víbora de Haly (Gloydius halys) han demostrado propiedades anticancerígenas que inhiben el crecimiento del tumor. Estas toxinas bloquean la interacción célula a célula, junto con la comunicación célula-matriz y la transmisión de señales [88], deteniendo el crecimiento de tumores metastásicos y sólidos. Este efecto también se ha encontrado en venenos de otras víboras no estrechamente relacionadas [88,89]. De forma similar, se ha demostrado que las desintegrinas contortrostatina y vicrostatina extraídas de Akistrodon contortrix (víbora cabeza de cobre), Echis carinatus (Forsa) y Eristicophis macmahoni (víbora del desierto de McMahon) impiden la movilidad, invasión, metástasis y angiogénesis tumoral de las células cancerosas [90,92,93,96,97].
L-aminoácido oxidasas (LAAO)
Se ha evidenciado que las toxinas LAAOs que se encuentra en los venenos de Ophiophagus hannah (cobra real) y Deinagkistrodon acutus (víbora mocasín china) inducen la reducción de la proliferación celular y apoptosis [88,89,96]. De manera similar, las toxinas CR-LAAO del veneno de Calloselasma rhodostoma (víbora malaya) han mostrado propiedades anticancerígenas provocando daños en el ADN de células cancerosas. De forma similar, la toxina BjussuLAAO-II de Bothrops jararacussu (víbora jararacussu) tiene efectos genotóxicos y citotóxicos en las células cancerosas [84,85,94].
Varios estudios han descrito que las LAAOs de algunas especies de los géneros Bothrops, Calloselasma, Cerastes, Crotalus, Cryptelytrops, Bungarus y Micrurus podrían inducir autofagia, apoptosis y necrosis en células normales y cancerosas [84]. Adicionalmente, estas toxinas también pueden activar citocinas proinflamatorias, lo que induce la muerte de células cancerosas mediante la activación del sistema inmune [84,85,90,94].
Defensa antimicrobiana
Se ha reportado que varias toxinas de veneno serpiente tienen una actividad antimicrobiana muy eficaz. La defensina y catelicidina son los principales grupos de péptidos antimicrobianos naturales con potentes propiedades microbicidas contra bacterias, hongos y algunos virus. Las proteínas más comunes con actividad antimicrobiana son PLA2 y LAAO (ver Capítulo 5). Estas toxinas desestabilizan la membrana del microorganismo y producen concentraciones locales de H2O2 que son tóxicas para el microorganismo (Tabla 3). Las PLA2 y LAAO también han demostrado actividad contra bacterias grampositivas y gramnegativas, parásitos y otros virus [33,98–100].
Otras toxinas no enzimáticas, como las lectinas de tipo C, 3FTx y catelicidinas, se han reportado como compuestos con actividad antimicrobiana. Por ejemplo, la lectina de tipo C del veneno de Bothrops leucurus (cabeza de lanza de cola blanca) ha sido eficaz contra bacterias como Staphylococcus aureus, Enterococcus faecalis y Bacillus subtilis [101]. Asimismo, proteínas pertenecientes a la misma familia, aisladas del veneno del Bothrops jararacussu (víbora jararacussu), actúan contra S. aureus [102]. De manera similar, las lectinas de tipo C del veneno de Crotalus durissus (cascabel suramericana) han mostrado actividad contra Xanthomonas axonopodis y Clavibacter michiganensis (bacterias inductoras de cáncer) [103].
Algunas proteínas 3FTx también han sido reportadas como agentes antimicrobianos, específicamente aquellos aislados del veneno de cobras (género Naja). El modo de acción de estas toxinas es unirse a los lipopolisacáridos y al ácido lipoteicoico, los principales componentes de la pared bacteriana, [104,105].
Los principales péptidos con actividad antimicrobiana son las catelicidinas, los cuales han sido identificado en varios transcriptomas de serpientes. Estos péptidos tienen actividad frente a Acinetobacter baumannii la cual es multirresistente (MRAB) y S. aureus resistente a meticilina. El control de estas bacterias se ha convertido en un reto para los fármacos comercializados actualmente dado a su alta resistencia. Las catelicidinas derivadas de serpientes más estudiadas son las de Naja atra (cobra china), Bungarus fasciatus (krait rayado) y Ophiophagus hannah (cobra real) [106–110].Se hipotetiza que la acción microbiana de todas las toxinas anteriormente nombradas tiene como función ecológica bloquear la proliferación de microorganismos infecciosos presentes en las presas de las serpientes, facilitando así su ingesta.
Sistema hemostático
Agentes antitrombóticos
El desarrollo del Captopril®, así como el diseño de los agentes antitrombóticos, son los casos más exitosos de producción de fármacos a partir de venenos de serpiente (Tabla 2). Como se mencionó anteriormente, Eptiftibatide (Integrilin®, Millenium Pharmaceuticals) y Tirofiban (Agrastat®, Merck & Co) se obtuvieron a partir de desintegrinas de veneno de serpiente [20]. Estos medicamentos están disponibles para tratar el infarto agudo de miocardio, el síndrome coronario agudo y las intervenciones coronarias percutáneas (Tabla 2).
El fármaco equistatina, aislado del veneno de Echis carinatus (víbora de forsa) es un antagonista eficaz de la agregación plaquetaria inducida por fibrinógeno [111]. Esta desintegrina contiene un arreglo estructural RGD (ver Capítulo 5) y puede unirse a varias integrinas en el rango de nanomolar. Por lo tanto, el fármaco requiere cantidades mínimas para bloquear las integrinas e impedir la agregación plaquetaria. Sin embargo, considerando que la distancia entre la arginina (R) y el aspartato (D) es un determinante estructural de la actividad de estas toxinas, Merck & Co realizó algunas modificaciones, incluida la inserción de tirosina en el sitio de un 4- (4-piperidinil) grupo butilo en el extremo N-terminal y grupo (S)-butilsulfonilamino en el extremo C-terminal. Estas modificaciones llevaron a desarrollar un compuesto con una gran potencia, llamado MK-0383, conocido como Tirofiban, el cual puede inhibir la agregación plaquetaria (Tabla 3). Este fármaco se utiliza en el infarto agudo de miocardio, el síndrome coronario agudo y la intervención coronaria percutánea [112,113].
Del mismo modo, la barbourina, aislada del veneno de Sistrurus miliarius (cascabel pigmea centroamericana), presenta una alta capacidad para inhibir la agregación plaquetaria [114,115]. La barbourina tiene un motivo KGD, por lo cual, para llevarla a nivel de producto farmacológico final, requirió varias modificaciones hasta obtener el producto final llamado Eptiftibatide. Este fármaco requirió la ciclación del péptido y la derivación de la cadena lateral de lisina en el motivo KGD (Tabla 3). Este medicamento además de su capacidad antitrombótica, se usa en el infarto agudo de miocardio, el síndrome coronario agudo y la intervención coronaria percutánea [116,117].
Otro grupo de toxinas que tienen potencial como agentes antitrombóticos son las lectinas de tipo C que se unen a un receptor diferente en la superficie de las plaquetas (ver Capítulo 5). Por ejemplo, la vixapatina, aislada del veneno de la víbora otomana (Montivipera xanthina), tiene un gran potencial como inhibidor de la agregación plaquetaria al bloquear el receptor α2β1 [118]. Después de algunas modificaciones, esta toxina conduce al diseño del fármaco Vipegitide. Este ha demostrado la capacidad de antagonizar dicho receptor en la plaqueta, y se considera un nuevo tipo de plantilla de un agente antitrombótico derivado de los venenos de serpiente [119].
Usos biomédicos de las toxinas del veneno de serpiente en el sistema hemostático
Debido a la acción de las toxinas del veneno de serpiente sobre los factores de coagulación de la sangre y las plaquetas, pueden utilizarse como herramientas para estudiar la coagulación, así como emplearse en estrategias en la clínica química como alternativas a los reactivos convencionales. Por ejemplo, la enzima batroxobina (Reptilase®) del veneno de serpiente similar a la trombina, aislada del veneno de la mapaná (Bothrops atrox) se usa para determinar el tiempo de reptilasa (un análisis de sangre que se usa para detectar deficiencias o anomalías en el fibrinógeno, especialmente en casos de contaminación por heparina). Esta prueba suele realizarse para confirmar o descartar la sospecha de disfibrinogenemias o alternativa en muestras que contienen heparina [120]. Otra proteína con importante actividad es Ancrod, aislada del veneno de la víbora malaya (Calloselasma rhodostoma). Ambas toxinas, ya sea Reptlase o Ancrod, también se emplean en el ensayo de antitrombina III, para lo cual el plasma debe estar libre de fibrinógeno, y no se puede agregar trombina porque su reacción con la antitrombina III podría interferir con la prueba [121].
Otras proteínas de los venenos de serpientes de las familias Viperidae y Elapidae con potencial biomédico son los activadores de protrombina [122]. Estas toxinas tienen varias aplicaciones, incluida la preparación de meizotrombina (uno de los principales productos de la activación de la protrombina), producción no enzimática de trombina o meizotrombina, y en estudios de hidrólisis de protrombina [120,123–125]. Por ejemplo, la producción de meizotrombina por ecarin, un SVMP aislado del veneno de Echis carinatus (víbora de forsa) se utiliza como herramienta de diagnóstico para el anticoagulante del lupus [126]. Además, el tiempo de cambio de la ecarin también se utiliza para monitorizar las propiedades anticoagulantes del etexilato de dabigatrán, fármaco indicado en el evento tromboembólico venoso [127].
Además, los venenos de serpiente también contienen activadores de otros factores de coagulación de la sangre, como los factores V y X. Por ejemplo, el veneno de Daboia russelli (víbora de Russell) contiene RVV-V y RVV-X, toxinas que activan el factor V y el factor X, respectivamente. Por lo tanto, RVV-V se utiliza en ensayos de rutina del factor V; y la RVV-X se utiliza en pruebas para cuantificar el factor X de la cascada de la coagulación para diferenciar las deficiencias de los factores VII y X [128–131].
Tabla 3. Fármacos o principios activos de venenos de serpiente utilizados en ensayos clínicos.
|
Medicamentos
|
Actividad
|
Fase
|
Sistema
|
Indicación
|
Origen
|
Referencia
|
|
Alfimeprasa
|
Actividad trombolítica
|
Ensayo clínico
|
Cardiovascular
|
Oclusión arterial periférica aguda
|
Agkistrodon contortrix
|
[29,32,35]
|
|
Viprinex (Ancrod)
|
Agente desfibrinogenante
|
Ensayo clínico
|
Cardiovascular
|
Accidente cerebrovascular isquémico agudo
|
Calloselasma rhodostoma
|
[29,61,71]
|
|
Protac/protein C activator
|
Activador de proteína C
|
Ensayo clínico
|
Hemostásico
|
Diagnóstico clínico del trastorno hemostásico
|
Agkistrodon contortix
|
[29,33,35]
|
|
Ecarina
|
Activador protrombina
|
Ensayo clínico
|
Hemostásico
|
Diagnóstico
|
Equis carinatus
|
[32,33,71]
|
|
Vivostat
|
Enzima similar a la trombina de veneno de serpiente/Serina proteasa
|
Ensayo clínico
|
Hemostásico
|
Anticoagulante
|
Bothrops moojeni
|
[33,35,74]
|
|
Anfibatida
|
Proteína tipo lectina tipo C/proteinasa
|
Ensayo clínico
|
Hemostásico
|
Actividades trombolíticas y antitrombóticas
|
Deinagkistrodon acutus
|
[29,32,74]
|
|
Cenderitida
|
Péptido natriurético e hipotensor
|
Ensayo clínico
|
Cardiovascular
|
Hipertensión
|
Dendroaspis angusticeps
|
[29,32,71]
|
|
RPI-MN/ RPI- 78M
|
Actividades antivirales, neuromoduladores y analgésicas
|
Ensayo clínico
|
Defensa antimicrobiana, sistema nervioso
|
Cepas de VIH resistentes a fármacos, tratamiento de esclerosis múltiple, distrofia muscular, miastenia gravis y esclerosis lateral amiotrófica
|
Naja kaouthia
|
[29,32,35]
|
|
Suling
|
Enzima tipo trombina de veneno de serpiente/ serina de proteasa
|
Ensayo clínico
|
Hemostásico
|
Anticoagulante
|
Deinagkistrodon acutus
|
[29,61,71]
|
|
Contortrostatin
|
Desintegrina
|
Estudios preclínicos
|
Hemostásico, cardiovascular
|
Inhibe la agregación plaquetaria
|
Agkistrodon contortrix
|
[29,33,35]
|
|
Textilinina
|
Inhibidor de la serina proteasa tipo Kunitz/inhibidor de la plasmina
|
Estudios preclínicos
|
Hemostásico
|
Agente antihemorrágico
|
Pseudonaja textilis
|
[32,33,71]
|
|
Haempatch
|
Activador de protrombina / Proteína similar al factor Xa
|
Estudios preclínicos
|
Hemostásico
|
Agente coagulante
|
Pseudonaja textilis
|
[33,35,74]
|
|
CoVase
|
Pro-factor de coagulación/proteína similar al factor Va
|
Estudios preclínicos
|
Hemostásico
|
Agente hemorrágico no compresible
|
Pseudonaja textilis
|
[29,32,74]
|
|
Vicrostatina
|
Desintegrina quimérica
|
Potencial terapéutico
|
Hemostásico, cardiovascular
|
Antitrombótico/ inhibe la agregación plaquetaria
|
Echis carinatus, Agkistrodon contortrix
|
[29,32,71]
|
|
Salmosina
|
Desintegrina
|
Potencial terapéutico
|
Cáncer, Hemostásico
|
Hemostasia dependiente de plaquetas/ bloqueante de integrina (αvβ3) / agente anticancerígeno
|
Gloydius halys
|
[33,35,74]
|
|
Oxynor
|
Neurotoxina presináptica (β-taipoxina) /Actividad mitógenica
|
Potencial terapéutico
|
Cáncer
|
Mitógeno/ cicatrización de heridas
|
Oxyuranus scutellatus
|
[29,32,74]
|
|
Prohanina
|
α-Neurotoxina (Hannalgesin)/ Actividad antinociceptiva/ efecto analgésico
|
Potencial terapéutico
|
Sistema nervioso
|
Actividad antinociceptiva/ efecto analgésico
|
Ophiophagus hannah
|
[29,32,71]
|
3. Potencial farmacológico y biomédico de los venenos de serpientes colombianas
Históricamente, los usos médicos o farmacológicos de los venenos de las especies de serpientes colombianas han sido poco explorados. A pesar de la asombrosa diversidad de serpientes venenosas que habitan en los ecosistemas del país, así como su alto endemismo (ver Capítulo 1); solo en las últimas décadas las investigaciones nacionales y extranjeras han centrado su interés al reconocer el enorme potencial farmacéutico de sus venenos [132].
Estos esfuerzos han contribuido al conocimiento de las especies endémicas colombianas, las manifestaciones clínicas que causan sus envenenamientos, así como en el desarrollo de nuevos agentes terapéuticos. No obstante, los estudios sobre usos médicos o farmacológicos en el país siguen siendo incipientes, aunque extraordinariamente prometedores. Como se explicó anteriormente, se han encontrado muchas indicaciones dentro de los usos farmacológicos de venenos de serpientes que habitan en Colombia. En esta sección presentamos una revisión detallada de los usos terapéuticos de las toxinas presentes en los venenos de serpientes colombianas (Tabla 4).
Vipéridos
Los venenos de serpientes de especies colombianas de la familia Viperidae son ricos en PLA2s, SVMPs, SVSPs; y tienen cantidades moderadas de LAAO, péptidos potenciadores de bradiquinina, desintegrinas y lectinas de tipo C (ver Capítulos 3 y 5). Por lo cual, es factible que los venenos tengan potencial antibacteriano, antiparasitario, anticancerígeno, antitrombótico, entre otras actividades. De ahí que, las PLA2 de Porthidium nasutum (patoco), Bothrops asper (mapaná), y poblaciones colombianas de Crotalus durissus (cascabel suramericana) hayan mostrado en varios estudios actividades antibacterianas y antiplasmodial, respectivamente [133,134].
Las LAAO de Crotalus durissus y Bothriechis schlegelii (víbora de pestaña) también han demostrado actividad antibacteriana [135,136]. El veneno de Bothrocophias myersi (víbora cabeza de sapo chocoana) puede tener un gran potencial como fuente de antimicrobianos, ya que presenta la mayor cantidad de PLA2 en comparación con los demás venenos de los vipéridos colombianos caracterizados (ver Capítulo 5) [137]. Sin embargo, para todos los casos mencionados anteriormente (PLA2 y LAAO), se necesitan más estudios que brinden información sólida sobre el mecanismo de acción de estas toxinas sobre las bacterias y su toxicidad. No obstante, se cuenta con indicios que las PLA2 de B. asper no son letales para los ratones en dosis tan altas como 15000 µg/kg, indicando baja toxicidad [134].
Entre todos los venenos de vipéridos colombianos caracterizados hasta ahora, el veneno de Lachesis acrochorda (verrugoso chocoano) presenta la mayor cantidad de BPP y SVSP en su composición. Por lo tanto, el veneno de esta especie surge como una fuente potencial de nuevos fármacos antihipertensivos. Particularmente, algunos tipos de SVSP son enzimas similares a la calicreína responsables de liberar bradicinina y potenciar las acciones hipotensoras de otras toxinas que pueden considerarse fuentes de moléculas antihipertensivas [138]. Estas proteínas ya han sido aisladas en Brasil del veneno del verrugoso amazónico (Lachesis muta), su especie hermana [139]. De manera que el veneno del verrugoso chocoano posee un alto potencial en bioprospección, por lo cual recomendamos enormemente realizar estudios que ayuden a comprender los mecanismos de acción de sus toxinas.
A excepción de Crotalus durissus y Lachesis acrochorda, los venenos de las demás víboras colombianas poseen desintegrinas como parte de su composición. Como se describió anteriormente, las desintegrinas se usan como guía para desarrollar agentes antitrombóticos. Sin embargo, actualmente no hay estudios que incluyan víboras colombianas en la exploración de desintegrinas en aplicaciones farmacológicas. Por lo tanto, se debe realizar estudios de aislamiento y caracterización de este tipo de proteínas de los venenos de serpientes colombianas para aumentar nuestro conocimiento sobre ellas y esclarecer su potencial farmacológico.
Asimismo, las lectinas tipo C, presentes en todos los venenos de los vipéridos colombianos caracterizados hasta el momento, no han sido estudiadas farmacológicamente en Colombia. Sugerimos que los venenos con mayor potencial son aquellos que contienen altas concentraciones de este tipo de toxinas. Por ejemplo, el veneno de Bothrops punctatus (rabo de chucha) posee un 16,7% de lectinas tipo C [140] y B. asper solo un 8,54% [141]. Las toxinas de lectina tipo C inhiben la agregación plaquetaria y pueden ser útiles en el desarrollo de fármacos antitrombóticos.
Otro caso importante es el Nasulysin-1, un SVMP aislado del veneno de Porthidium nasutum. Esta toxina provoca una actividad inductora de apoptosis específica (muerte celular programada) en células Jurkat y K562, célula T de leucemia linfocítica aguda (LLA), así como en modelos celulares de leucemia mieloide crónica (LMA), sin afectar la viabilidad de las células de linfocitos humanos. Además, este SVMP activa la caspasa-3 y el factor inductor de apoptosis (AIF) [142]. Sin embargo, se necesitan más estudios para obtener información sobre la toxicidad de Nasulysin-1 y los determinantes estructurales de la actividad citotóxica para evaluar su potencial real como medicamento anticancerígeno.
En particular, entre las víboras colombianas, Crotalus durissus la única serpiente de cascabel presente en el país, posee crotoxina una neurotoxina exclusiva de las serpientes de cascabel, construida a partir de una subunidad básica que es PLA2 (CB) y una subunidad ácida (CA) formada por tres péptidos (α, β y γ) unidos por siete enlaces disulfuro (ver Capítulo 5). Esta toxina actúa como acompañante de la subunidad CB [143]. La crotoxina, tiene actividad antitumoral demostrada en cultivo celular y en pacientes con tumores sólidos que son refractarios a la terapia convencional [144–147]. Un estudio reciente propuso que la crotoxina pudiera ser una herramienta útil para evitar el desarrollo de trombosis al reducir los niveles de proteínas procoagulantes y aumentar los de proteínas anticoagulantes [148]. Finalmente, la cadena α de la subunidad CA de la crotoxina tiene actividad analgésica mediada por varios mecanismos. Por todas estas razones, Crotalus durissus entre todas las serpientes colombianas, es una de las especies que presenta el mayor potencial farmacológico.
Aunque, los estudios sobre usos médicos o farmacológicos de los venenos son incipientes en Colombia, podemos encontrar varias moléculas con actividad terapéutica comprobada derivadas de especies venenosas que habitan en los ecosistemas colombianos (Figura 3). Los efectos e indicaciones de estas moléculas se mencionan a continuación, abarcando especies médicamente importantes de vipéridos, elápidos y colúbridos.
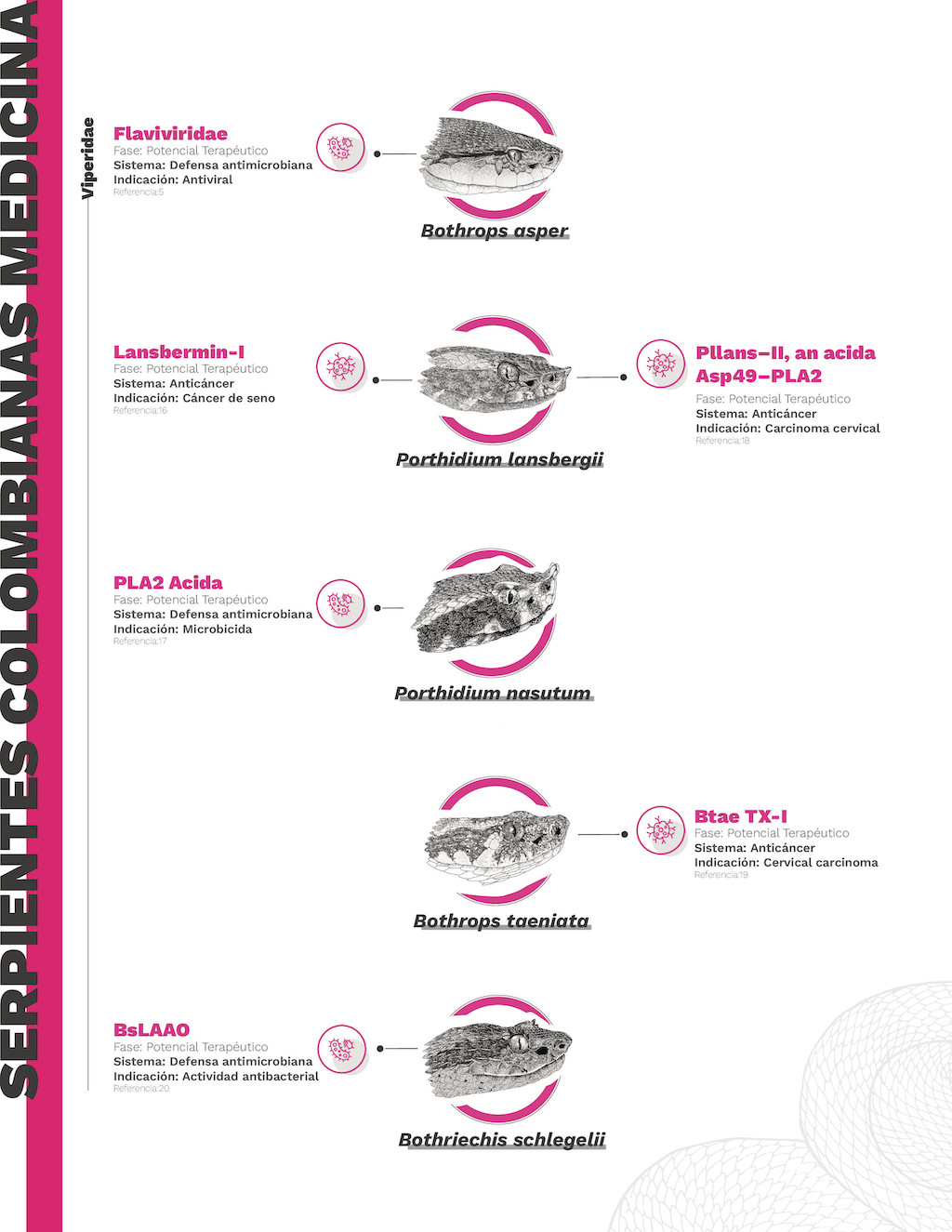
Figura 4. Infografía de los usos farmacológicos de los compuestos activos derivados de serpientes colombianas
Bothrops atrox.— La batroxicidina ha demostrado actividad antibacteriana. Esta molécula tiene actividad antichagas al inducir necrosis y apoptosis a Trypanosoma cruzi (parásito que causa la enfermedad de Chagas). Su acción se comparó con los fármacos actualmente disponibles (benzinidazol), mostrando una mayor eficacia incluso contra la cepa de T. cruzi resistente a benzinidazol. Este hallazgo convierte a la batroxicidina en una molécula candidata para tratar la enfermedad de Chagas y parásitos sanguíneos similares [149].
Los péptidos de baja masa molecular Ba-V del veneno de B. atrox tienen efectos neuroprotectores. Estos péptidos tienen potencial terapéutico para enfermedades neurodegenerativas como el Parkinson y el Alzheimer al inhibir la transición de la permeabilidad mitocondrial, presumiblemente al alterar el flujo de calcio. Este proceso juega un papel importante en la fisiopatología de las enfermedades neurodegenerativas, y la inhibición de esta vía por parte de Ba-V podría prevenir la muerte celular cerebral [150]. Adicionalmente, una molécula similar, la Ba-IV, ha mostrado efectos neuroprotectores en enfermedades neurodegenerativas como la enfermedad de Parkinson. El Ba-IV inhibe las proteasas apoptóticas como la caspasa-9 y la caspasa-3 que previenen la muerte celular en las neuronas dopaminérgicas, el principal proceso presente en la enfermedad de Parkinson. Por tanto, Ba-V y Ba-IV podrían permitir prevenir la progresión de enfermedades neurodegenerativas [151].
Bothrops asper.— A partir del veneno de B. asper se han aislado dos moléculas denominadas Mt-I y Mt-II, isoformas similares a PLA2. Ambos han sido probados como posibles medicamentos para enfrentar enfermedades por flavivirus (fiebres hemorrágicas virales). Debido a su actividad enzimática, las toxinas Mt-I y II podrían permeabilizar la envoltura del virus, explicando su actividad antiviral, sin embargo, su mecanismo de acción aún no se conoce con precisión. Particularmente, Mt-I tiene una fuerte actividad antiviral, mientras que Mt-II no muestra los mismos resultados. Estos hallazgos podrían conducir a un tratamiento eficaz contra flavivirus como causante del dengue [152].
Otras toxinas de los venenos de B. asper con potencial terapéutico son las colombienasas I y II, y la batroxasa. Estos podrían ser útiles como agentes trombolíticos utilizados en enfermedades cardiovasculares. Estas enzimas tienen varias actividades biológicas entre ellas se destacan las hemorrágicas, fibrinogenolíticas, proteolíticas, hemolíticas, edematogénicas y citotóxicas. Por ejemplo, las toxinas colombienasas I y II degradan el fibrinógeno sin activar el sistema fibrinolítico (plasminógeno/plasmina) [153]. Además, la batroxasa modula las células proinflamatorias en la sangre humana e induce efectos citotóxicos en las células sanguíneas tumorales. Esta actividad podría regular la proliferación de células cancerosas presentes en la leucemia, y de esta molécula podría derivarse un nuevo tratamiento [154]
Bothrops taeniatus.— La actividad enzimática de Btae TX-I, una toxina de la familia de las PLA2 aislada del veneno de B. taeniatus,tiene propiedades anticancerígenas similares a las observadas en otras toxinas PLA2 presentes en venenos de sus congéneres (B. asper y B. atrox). Además, la Btae TX-I también presenta potencial como fármaco contra el cáncer, aunque sus mecanismos de acción aún están por ser descubiertos [155].
Crotalus durissus.— La serpiente de cascabel suramericana Crotalus durissus es una de las especies colombianas más prometedoras en la exploración farmacológica de nuevos fármacos. Actualmente, se han evaluado al menos cinco toxinas con usos farmacológicos. La toxina polipeptídica crotamina aislada del veneno de C. durissus ha mostrado efectos antiparasitarios, especialmente en infecciones por Plasmodium falciparum (causante de la malaria). La crotamina logra ingresar al núcleo del parásito, alterando la regulación del pH modificando la acidez ambiental, lo que provoca cambios en la membrana del parásito, y finalmente su muerte. Este efecto se logra sin afectar las células sanguíneas sanas, lo que demuestra el excelente potencial para tratar la malaria [156]. Adicionalmente, este mismo mecanismo se ha estudiado como actividad anticancerígena frente al melanoma, cáncer de páncreas y carcinoma, en los que la crotamina ha mostrado eficacia [157].
La citotoxina (Cdt), una molécula PLA2 del veneno de C. durissus tiene actividad antiviral. Esta toxina ha sido descrita como útil contra el virus del sarampión al bloquear los receptores celulares que el virus necesita para su adsorción. Sin embargo, se necesita más investigación sobre su efectividad, ya que solo se demostró que esta molécula evita que el virus ingrese a la célula después de la infección, pero no una acción letal sobre el virus. Sin embargo, este hallazgo puede dilucidar nuevos mecanismos de acción para los fármacos antivirales [158].
Por otro lado, al aislar la subunidad CB de la crotoxina, se ha demostrado que esta fracción de la toxina regula el flujo de entrada del canal de cloro de la membrana celular. Por tanto, la crotoxina podría dar lugar a aplicaciones médicas como el tratamiento de la fibrosis quística, una enfermedad caracterizada por una mutación genética que conduce a alteraciones en la regulación de los canales de cloro. Estas alteraciones conducen daños sistémicos y limita considerablemente la esperanza de vida del paciente [159].
Asimismo, se ha descrito la actividad antibacteriana de la crotalicidina. Esta molécula se evaluó como antibacterial contra infecciones por Escherichia coli y Pseudomonas aeruginosa. La crotalicidina provoca la ruptura de la membrana celular de la bacteria que conduce a la muerte bacteriana. Una ventaja de la crotalicidina contra las infecciones es que su concentración efectiva no daña las células humanas sanas. Este hallazgo representa una excelente noticia para la resistencia bacteriana a los medicamentos actuales; y en el futuro, podría ayudar a producir una nueva generación de antibióticos [157,160]. Adicionalmente, se encontró que las PLA2 de Crotalus durissus también tiene actividad antiviral, especialmente para el virus del dengue. El mecanismo está relacionado con la actividad enzimática de estas toxinas, provocando la degradación de la envoltura del virus, exponiendo su RNA, facilitando su degradación por las enzimas RNasa [161].
Finalmente, se ha descrito otro potencial terapéutico de una toxina del veneno de C. durissus. El péptido crotalfina tiene una actividad antinociceptiva que podría ser importante en el tratamiento del dolor crónico, como el que presentan pacientes con cáncer. Además, los efectos de la crotalfina son inducidos por los receptores opioides kappa y delta, lo que conduce a un manejo del dolor más duradero en comparación con la morfina. En consecuencia, esta molécula podría ser beneficiosa para evitar la administración de fármacos opioides, previniendo el síndrome de abstinencia [162]. Además, una molécula similar, la crotapotina, también ha mostrado efectos analgésicos y está siendo estudiada para el dolor en encefalomielitis y esclerosis múltiple, estableciéndola como una molécula prometedora para tratar este tipo de dolor [163].
Lachesis muta. – El péptido llamado LmrBPP9 se sintetizó a partir del veneno verrugoso amazónico. Esta molécula tiene actividad inhibidora de la ECA como el BPPS utilizado para crear el captopril. Además, LmrBPP9 reduce eficazmente la presión arterial, de forma comparable al captopril. Estos resultados ofrecen nuevas fuentes de BPPS para el diseño medicamentos para tratar la hipertensión [164].
Complejo de especies del género Porthidium. – Actualmente, se ha descubierto que dos componentes del veneno de Porthidium lansbergii (patoco)tienen actividad anticancerígena. La Pllans-II (un tipo de PLA2) se evaluó contra el cáncer de cuello uterino y mostró efectividad sin dañar los tejidos sanos [165]. También, la desintegrina Lansbermin-I aislada del veneno de P. lansbergii presenta actividad anticancerígena en células de cáncer de mama. Esta toxina afecta la angiogénesis y la migración de células de cáncer de mama. Estas moléculas ofrecen nuevas alternativas para la investigación y creación de nuevos fármacos para el tratamiento del cáncer [91,166].
Asimismo, una toxina PLA2 ácida aislada de Porthidium nasutum demostró actividad antibacteriana. Al igual que otras toxinas PLA2, el mecanismo también implica la ruptura de la membrana bacteriana, lo que la convierte en un potente bactericida, añadiéndola a las moléculas con potencial para crear nuevas generaciones de antibióticos [133].
Complejo de especies Bothriechis schlegelii.— A pesar de que esta especie arborícola se ve con frecuencia en diversas elevaciones de los Andes colombianos (0-3200 msnm), su veneno ha sido pobremente estudiado y su potencial farmacológico es poco conocido. No obstante, del veneno de B.schlegelii la toxina BsLAAO presenta una potente actividad antibacteriana similar a las toxinas LAAO presentes en otros vipéridos. Principalmente, se estudiaron sus efectos sobre las infecciones provocadas por Staphylococcus aureus y Acinetobacter baumanni. Esta última es una de las bacterias con mayor resistencia a los antibióticos, causando una alta tasa de mortalidad. Por tal razón, los compuestos derivados del veneno de B.schlegelii representan una alternativa muy atractiva para crear una nueva generación de antibióticos que ayuden a controlar la resistencia a los antibióticos y reducir las tasas de mortalidad [167].
Elápidos
Los venenos de serpientes coral han sido menos estudiados que los venenos de víboras. Esto debido a la dificultad de obtener muestras de veneno con un volumen adecuado extraído por espécimen, que permita la purificación de las proteínas. Solo se ha caracterizado el proteoma y composición del veneno de tres especies colombianas: Micrurus mipartitus, M. dumerillii, y M. helleri [168–170]. Todos estos venenos tienen cantidades significativas de 3FTx, PLA2 y LAAO, lo que los convierte en fuentes potenciales de compuestos que podrían ser activos contra enfermedades cardiovasculares, infecciones antibacterianas, antivirales y antiparasitarias, así como medicamentos analgésicos. Aunque, se han purificado varias toxinas de los venenos de M. mipartitus y M. dumerillii,no se han evaluado sus actividades analgésicas o de modulación de los canales iónicos; quizás estas actividades sean similares a otras 3FTx que se han estudiado en los elápidos asiáticos.
Micrurus helleri.— La exploración del potencial farmacológico del veneno de M. helleri da como resultado el aislamiento de una molécula de PLA2 denominada MlV. Este compuesto tiene actividad antinociceptiva, demostrando un efecto superior en comparación con los fármacos convencionales [morfina (opioide) e indometacina (AINE)] sin afectar el sistema locomotor. Los efectos de MlV se manifiestan mediante el bloqueo de los receptores opioides. Este descubrimiento brinda una excelente oportunidad para crear nuevos fármacos para tratar el dolor crónico [164].
Otra molécula de PLA2 aislada del veneno de M. helleri es la lemnitoxina. Esta toxina posee actividades miotóxicas, citotóxicas, proinflamatorias y anticoagulantes. Este último efecto ha sido estudiado debido al potencial terapéutico para diseñar nuevos fármacos anticoagulantes [171].
Micrurus mipartitus.— Esta es la especie de coral más común que habita en elevaciones bajas a moderadas de los Andes colombianos (400-2000 msnm). A pesar de esto, se han realizado pocos estudios farmacológicos. Exploraciones recientes han aislado una toxina de la familia de proteínas LAAO con una potente actividad bactericida contra dos bacterias (Staphylococcus aureus y Escherichia coli) [172]. Además, se han aislado y caracterizado varias toxinas de PLA2 no antes conocidas del veneno M. mipartitus,pero aún no se ha evaluado su potencial farmacológico [173].
Micrurus obscurus.— Actualmente, no se ha explorado el potencial farmacológico de venenos de serpientes M. obscurus de poblaciones colombianas. Sin embargo, se aisló una toxina denominada MsPLA2 que presenta una actividad tipo fosfolipasa del veneno de su especie hermana M. spixii de poblaciones brasileñas [174]. La MsPLA2 demostró actividad similar a la observadas en las PLA2 provenientes de venenos de especie del género Bothrops. Además, los estudios citotóxicos de MsPLA2 han demostrado actividades y efectos microbicidas y antitumorales contra P. falciparum y cáncer de hígado. Aunque sus efectos microbicidas y antitumoral han sido demostrados, su efectividad no supera la de los medicamentos actualmente disponibles. Sin embargo, la comprensión de sus mecanismos de acción puede ayudar a comprender los procesos biológicos de estas patologías y generar potencialmente nuevas moléculas que ayuden a combatirlas [175].
Colubridae
Pocos venenos de colúbridos colombianos han sido caracterizados por su contenido de toxinas utilizando técnicas proteómicas (ver Capítulo 4). Sin embargo, en los últimos años se ha generado conocimiento esencial para la bioprospección de toxinas de colúbridos [p. ej., Leptodeira annulata (falsa mapaná), Erythrolamprus bizona (falsa coral) y Pseudoboa neuwiedii (candelilla)] [176–178]. Desde el punto de vista farmacológico, las toxinas de colúbridos muestran un gran potencial como compuestos citotóxicos, antibacterianos, antivirales y antiparasitarios debido a su composición significativa de actividades PLA2 y SVMP. Además, el veneno de L. annulata muestra una fuerte actividad fibrinógena y fibrinolítica que sugieren la presencia de SVMPs, lo que conduce a moléculas para el desarrollo de agentes trombolíticos [178]. Sin embargo, su potencial farmacológico aún no ha sido evaluado.
Asimismo, se han caracterizado las toxinas 3FTx en colúbridos las cuales no son tóxicas para las células humanas. Como se explicó anteriormente, las 3FTx poseen varias actividades biológicas aplicables al uso médico. Sin embargo, se necesitan más estudios para explorar sus usos farmacológicos [179]. Algunos ejemplos de toxinas aisladas de colúbridos suramericanos evaluadas farmacológicamente son las moléculas H. t. texana y T.b. Lambda aislada de Philodryas baroni, P. olfersii, y P. patagoniensis. Estas toxinas pertenecen a la familia de las PLA2 y presentan actividad antileishmania. Aunque no son tan potentes como los fármacos actualmente disponibles (anfotericina B), representan nuevas oportunidades de fármacos contra la Leishmania [180].
En conclusión, el potencial terapéutico de los venenos de las serpientes colombianas es inmenso y puede ayudar a diseñar nuevos tratamientos para múltiples patologías. El uso de tecnologías «ómicas» permitirá identificar más moléculas potenciales en los venenos de serpientes de Colombia. Sin embargo, debemos seguir estudiando los componentes del veneno de manera convencional, mientras se puedan realizar tecnologías «ómicas» de forma masiva y a un costo razonable para los investigadores colombianos.
En este capítulo se presentó el potencial biomédico y farmacológico del veneno de serpientes que habitan en Colombia. Debido a la naturaleza compleja de estos venenos y los hallazgos descubiertos hasta ahora, está claro que son posibles muchos más descubrimientos de moléculas que pueden llevar al diseño de nuevos tratamientos y medicinas. Sin embargo, se necesitan más recursos para aumentar nuestro conocimiento sobre los venenos de serpientes colombianas y establecer una estrategia bioprospección clara de estas sustancias.
Tabla 4. Moléculas con potencial terapéutico derivadas de venenos de serpientes colombianas.
|
Familia
|
Especies
|
Principio activo
|
Mecanismo
|
Sistemas
|
Indicación
|
Referencia
|
|
Viperidae
|
Bothrops atrox
|
Batroxicidin
|
Induce necrosis y apoptosis en T. cruzi
|
Defensa antimicrobiana
|
Antichagas (T. cruzi)
|
[149]
|
|
Bothrops asper
|
Colombianasas
|
Degrada el fibrinógeno, pero no activa el sistema fibrinolítico (plasminógeno/plasmina)
|
Cardiovascular/Hemostásica
|
Terapia fibrinolítica.
|
[153]
|
|
Bothrops atrox
|
Batroxase
|
Citotoxicidad en células sanguíneas tumorales
|
Anticáncer
|
Leucemia.
|
[154]
|
|
Bothrops atrox
|
Ba-V
|
Inhibe la transición de la permeabilidad mitocondrial, lo que presumiblemente altera el flujo de calcio
|
Sistema nervioso
|
Potencial neuroprotector
|
[150]
|
|
Bothrops asper
|
Mt–I
|
Permeabilizar la envoltura del virus debido a la actividad enzimática.
|
Defensa antimicrobiana
|
Antiviral
|
[152]
|
|
Bothrops atrox
|
Ba-IV
|
Inhibición de proteasas apoptóticas como caspasa-9 y caspasa-3 que previene la muerte celular en neuronas dopaminérgicas
|
Sistema nervioso
|
Enfermedad de Parkinson
|
[151]
|
|
Crotalus
durissus
|
Crotamina
|
Alteración del equilibrio de H+ y perturbación de la membrana
|
Defensa antimicrobiana / Actividad anticáncer
|
Malaria / Melanoma, cáncer de páncreas y carcinoma.
|
[149,156]
|
|
Crotalus
durissus
|
Cdt
|
Bloquea los receptores en la membrana celular, impidiendo la absorción del virus
|
Defensa antimicrobiana
|
Actividad antiviral
|
[158]
|
|
Crotalus
durissus
|
Crotalicidina
|
Ruptura de la membrana celular bacteriana
|
Defensa antimicrobiana
|
Actividad antibiótica
|
[157,160]
|
|
Crotalus
durissus
|
Fosfolipasa A2
|
Degradación de la envoltura del virus, exponiendo el ARN del virus
|
Defensa antimicrobiana
|
Actividad antiviral (dengue)
|
[161]
|
|
Crotalus
durissus
|
Subunidad CB de crotoxina
|
Regulación de los canales de Cl-
|
Sistema respiratorio
|
Fibrosis quística
|
[159]
|
|
Lachesis muta
|
LmrBPP9
|
Inhibición de la ECA
|
Sistema cardiovascular
|
Hipertensión
|
[164]
|
|
Porthidium lansbergii
|
Lansbermin-I
|
Actividad de desintegrina
|
Anticáncer
|
Células de cáncer de mama
|
[91,166]
|
|
Porthidium nasutum
|
PLA2 ácida
|
Actividad de fosfolipasa
|
Defensa antimicrobiana
|
Actividad antibiótica
|
[133]
|
|
Porthidium lansbergii
|
Pllans–II, ácida Asp49–PLA2
|
Actividad de fosfolipasa
|
Anticáncer
|
Carcinoma cervical
|
[182]
|
|
Bothrops
taeniata
|
Btae TX-I
|
Actividad de fosfolipasa
|
Anticáncer
|
Carcinoma cervical
|
[155]
|
|
Bothriechis schlegelii
|
BsLAAO
|
Actividad de LAAO
|
Defensa antimicrobiana
|
Actividad antibiótica
|
[167]
|
|
Elapidae
|
Micrurus spixii
|
MsPLA2-I
|
Actividad de fosfolipasa
|
Defensa antimicrobiana / anticáncer
|
Actividad antibiótica / antitumoral
|
[175]
|
|
Micrurus
helleri
|
Lemnitoxina
|
Actividad de fosfolipasa
|
Sistema cardiovascular
|
Efectos anticoagulantes
|
[171]
|
|
Micrurus helleri
|
MlV
|
Bloqueo de los receptores opioides
|
Sistema nervioso
|
Efectos antinociceptivos
|
[181]
|
Apéndice: Materiales y métodos
Métodos de búsqueda para la identificación de estudios: Búsquedas electrónicas
Las búsquedas se realizaron de abril a junio de 2021. Realizamos las búsquedas de la siguiente manera:
- Búsquedas semanales del Registro Cochrane Central de Ensayos Controlados (CENTRAL)
- Búsquedas semanales de MEDLINE (Ovid)
- Búsquedas semanales de Embase (Ovid)
- Búsquedas semanales de Scielo
- Búsquedas semanales de Scopus
- Búsquedas semanales de Google Scholar
- Búsquedas semanales de Clinicaltrial.gov
Las estrategias de búsqueda contenían las siguientes palabras clave y términos que se combinaron de diferentes formas utilizando los conectores booleanos AND OR: snake venoms, Bothrops atrox, Crotalus, Bothiechis, Porthidium, Lachesis, Micrurus, Xenodon, Leptophis, Erythrolamprus, Oxybelis, Helicops, Bothrocophias, Thamnodynastes, Leptodeira, Philodryas, and drug development.
Buscando otros recursos
Verificamos los estudios citados relevantes al revisar los informes identificados por las búsquedas electrónicas, así como las listas de referencias de cualquier revisión directamente relevante identificada. No se aplicaron restricciones de idioma o fecha y se incluyeron estudios independientemente del tipo de publicación (p. ej., resumen de congreso, entrada de registro de ensayo, artículo de revista, libro).
Realizamos búsquedas en marzo de 2021 e identificamos 1087 artículos para su posible inclusión. Además, identificamos un total de 320 artículos para la selección de títulos y resúmenes, de los cuales los expertos sugirieron 39 artículos, 46 estudios estaban duplicados y 40 se excluyeron por otras razones.
Obtuvimos el texto completo de los 234 artículos restantes para su inclusión. Los detalles del flujo de estudios para esta revisión se registran en un diagrama PRISMA en la Figura 4.
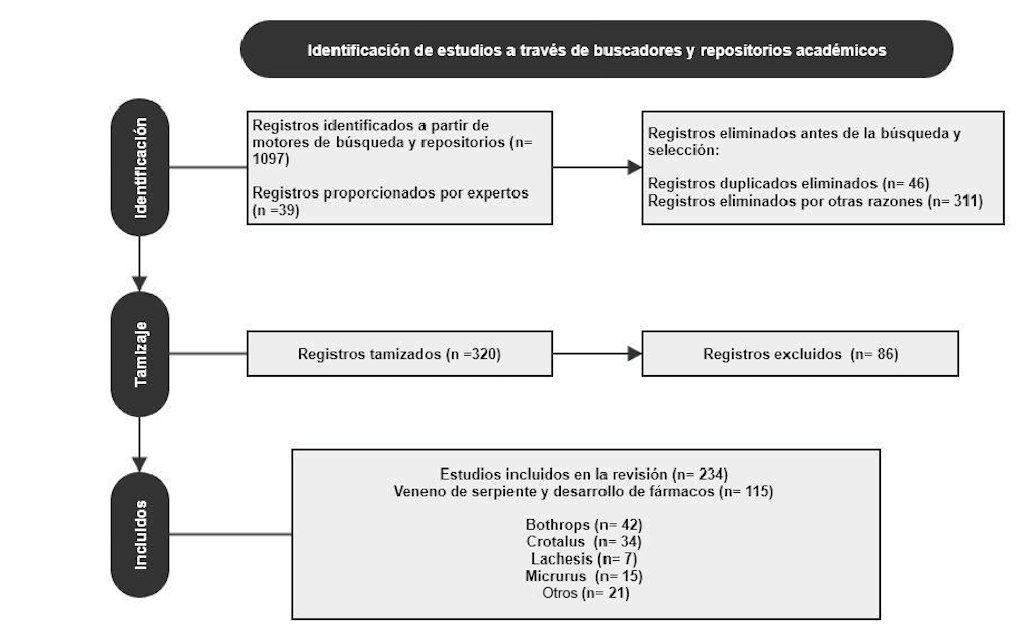
Figure 4. Flujo diagrama de la metodología PRISMA empleada.
