1. Tratamiento contra mordeduras de serpientes en Colombia
1.1 ¿Qué es un antiveneno?
Aunque no hay consenso sobre la definición exacta de un antiveneno, generalmente se entiende como un medicamento o tratamiento que contrarresta los efectos nocivos atribuidos a un veneno o enfermedad [1]. Los humanos han desarrollado antídotos específicos para defenderse contra estas sustancias, incluyendo el grupo de antivenenos también conocidos como sueros, antisueros y antiofídicos. Históricamente, los anticuerpos purificados (terapias basadas en inmunoglobulinas) han jugado un papel fundamental en la desactivación de toxinas de venenos específicos [2–4].
De acuerdo con el marco regulatorio colombiano para la obtención de registro sanitario para antivenenos está descrito en el Decreto 386 de 2018 [10], los antiofídicos se definen como «fracciones purificadas de inmunoglobulinas o fragmentos de inmunoglobulinas del plasma obtenidos de animales inmunizados con un veneno o una mezcla de venenos». Los antivenenos se preparan hiperinmunizando animales, como ovejas o caballos, por medio de la inoculación de pequeñas dosis de veneno que estimulan la producción de anticuerpos que desactivan toxinas. Los anticuerpos se obtienen extrayendo sangre del animal [3–5]. Una vez que los anticuerpos son aislados de una fracción purificada de sueros hiperinmunes de origen equino, se produce el antiofídico el cual es empleado como tratamiento terapéutico contra el envenenamiento causado por mordeduras de serpientes. El tratamiento consiste en administrar vía endovenosa estos anticuerpos los cuales neutralizan las toxinas del veneno. Este es el único modo de salvar la vida de las personas que accidentalmente han sido envenenadas [5,6,7].
Es importante reconocer que, aunque los antiofídicos son medicamentos esenciales para tratar los envenenamientos causados por serpientes, especialmente en países tropicales y subtropicales [8], desafortunadamente su distribución es inadecuada y muy limitada. Solo unos pocos países en latitudes tropicales cumplen con los estándares de calidad adecuados para fabricar estos medicamentos. Además, algunos países carecen de una regulación y control adecuados sobre su producción, lo que dificulta en gran medida la evaluación e intervención en el control de la calidad y efectividad de los antiofídicos [9].
1.2 Breve historia de la producción de antivenenos en el Instituto Nacional de Salud de Colombia
El Instituto Nacional de Salud (INS) es una entidad gubernamental científica y técnica que ha estado produciendo formalmente suero antiofídico polivalente desde la década de 1970 (ver Capítulo 7). Este suero se elabora a partir de plasmas hiperinmunes obtenidos mediante esquemas de inmunización estandarizados, utilizando venenos de especies de serpientes de importancia médica provenientes de las diferentes regiones biogeográficas de Colombia. La producción de antiofídicos cumple con el marco regulatorio vigente y aplicable [11].
Con base en los registros de producción del INS, las primeras extracciones de plasma hiperinmune de origen equino para la producción de antiofídicos datan del siglo XX a comienzos de los años 70’s. Durante este periodo, también se realizaron los primeros registros de la producción de plasma hiperinmune antirrábico. Posteriormente, en febrero de 1972, se registró la producción de plasmas hiperinmunes botrópicos y crotálicos, así como los primeros ensayos de producción de antiofídicos en el INS. En ese momento, hubo dificultades debido a la falta de experiencia en el manejo de venenos de serpientes, protocolos de inmunización, técnicas de titulación y procedimientos de producción. El primer lote consistió en aproximadamente 150 viales, pero debido a las dificultades, la producción fue interrumpida (ver Capítulo 7).
Dos años después, los médicos Augusto Corredor, Miguel Guzmán y Ernesto Barbosa del INS asistieron al Congreso de Medicina Tropical en Medellín. El Dr. Roger Bolaños, conocido por su amplia experiencia en la producción de antivenenos y el manejo de mordeduras de serpientes, también asistió al evento. Los médicos del INS contactaron al Dr. Bolaños a través de la Organización Panamericana de la Salud (OPS) para contratarlo como consultor para el laboratorio de sueros del INS. El Dr. Bolaños capacitó al equipo de producción conformado por Juan Manuel Rengifo, Guiomar Caicedo de Pardo y Carlos Cáceres en la elaboración de antivenenos (ver Capítulo 7). Produjeron exitosamente un lote experimental de plasma hiperinmune de caballos inmunizados con el veneno de Bothrops asper, el cual fue aprobado por su capacidad neutralizante. Esta victoria temprana permitió el desarrollo de nuevos protocolos de producción y esquemas de inmunización, consolidando así el inicio de la producción formal de antiofídicos en Colombia.
Posteriormente, Juan Manuel Rengifo y Guiomar Caicedo de Pardo recibieron capacitación complementaria en el Instituto Clodomiro Picado, donde adquirieron conocimientos sobre el manejo de serpentarios, esquemas de inmunización y procesos de producción. Como resultado de esta capacitación, el INS pudo fabricar el primer lote oficial de suero antiofídico polivalente (Lote No. 4) en su historia. Esta producción permitió comenzar oficialmente el 23 de junio de 1975. Actualmente, el INS trabaja con una diversidad de venenos (ver Capítulo 8). Los lotes previamente producidos No. 1, No. 2 y No. 3 se utilizaron para estandarizar la producción y el control de calidad para la producción de antiveneno botrópico-crotálico (AV B/C).
En 1975 se produjeron un total de 4.020 viales de suero antiofídico, consistiendo en 2.800 viales de suero antiofídico polivalente (SAP) y 1.220 viales de suero monovalente (SAM). De 1978 a 1990, el INS produjo un total de 120.472 viales de SAP y 20.334 viales de SAM. La producción de SAP alcanzó su punto más alto en la década en 1980 con 14.811 viales.
En 1993, la Ley 100 (de 1993) mediante el artículo 245 creó el Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA). Su principal objetivo es implementar políticas de vigilancia sanitaria y asegurar el control de calidad de medicamentos, productos biológicos, alimentos, bebidas, cosméticos, dispositivos médico-quirúrgicos y odontológicos, productos homeopáticos, naturales, generados por biotecnología, de aseo, higiene y limpieza de uso doméstico. En 1994, el Decreto 1290 estableció las funciones del INVIMA, las cuales incluyen la implementación de las políticas formuladas por el Ministerio de Salud de Colombia para la vigilancia sanitaria y el control de calidad de productos descritas en el artículo 245 de la Ley 100 de 1993, así como otras regulaciones pertinentes y recomendaciones de la Comisión Revisora mencionada en el artículo 9.
En 1995, el Decreto 677 reguló parcialmente el régimen de registro y licenciamiento, control de calidad y vigilancia sanitaria para medicamentos, cosméticos, preparaciones farmacéuticas basadas en recursos naturales, productos de aseo, higiene y limpieza y otros productos de uso doméstico. También se emitieron otras disposiciones sobre el tema. El artículo 2 define específicamente el medicamento como: «una preparación farmacéutica obtenida a partir de ingredientes activos con o sin sustancias auxiliares presentada en forma farmacéutica que se usa para la prevención, alivio, diagnóstico, tratamiento, cura o rehabilitación de la enfermedad». Los contenedores, etiquetas y empaques también se consideran partes integrales del medicamento, ya que garantizan su calidad, estabilidad y uso adecuado. Esta clasificación incluye los sueros o antivenenos.
En el año 2002, el Grupo de Sueros del INS desarrolló, produjo y entregó, 182 viales de suero poliespecífico panafricano al Centro Colaborador de la OMS para el Control de Antivenenos (WHO CCCA) en la Escuela de Medicina Tropical de Liverpool, demostrando su capacidad para fabricar suero antiofídico destinado en este caso al continente africano. Durante ese período, el INS enfrentó intermitencias en la producción, lo que llevó al Ministerio de Salud a emitir la resolución 122 de 2002, declarando una emergencia sanitaria para garantizar el abastecimiento de biológicos en el país, incluyendo vacunas y antivenenos. Ante esta situación, el Ministerio solicitó al Fondo Rotatorio de la OPS/OMS los recursos necesarios para reactivar la producción de antiofídicos, en cumplimiento de lo dispuesto en el párrafo b del artículo 96 del Decreto 677 de 1995.
En 2004, los accidentes ofídicos fueron incluidos como eventos de notificación obligatoria, por lo tanto, los casos comenzaron a ser reportados en el Sistema de Vigilancia Epidemiológica de Colombia (SIVIGILA). La Resolución 2934 (2004) declaró una emergencia sanitaria en el territorio nacional debido a la escasez de suero antiofídico por un período de seis meses. Sin embargo, la declaración de emergencia se extendió hasta el 31 de diciembre de 2010, ya que las condiciones que llevaron a su declaración no se superaron. Esta extensión se realizó de acuerdo con la resolución 2672 (2010) y las resoluciones modificatorias 613 y 5078 (2005), resolución 2325 (2006), resolución 2198 (2007), resolución 2413 (2008) y resolución 2206 (2009).
Desde 1991 a 2011, la producción de SAP acumuló 133.869 viales y 19.898 viales de SAM, con un máximo de 16.206 viales de SAP en 1998 (Figura 1). En 2005, se registró la producción final de SAM, resultando en un total de 1.662 viales. En 2014, el INS aumentó la capacidad de producción, logrando liberar aproximadamente 3.500 viales, así como establecer un tamaño estándar de lote de 9.500±500 viales de SAP. Además, en 2017, el INS llevó a cabo una actualización tecnológica para incrementar y mantener la capacidad de producción, asegurando la disponibilidad de SAP para el país.
A principios de la década de 2010, el INS buscó mejorar su producción de antiofídicos y ampliar la cobertura a especies de serpientes de importancia médica. Para ello, desarrollaron el antiofídico anticoral polivalente (PAA). El lote inicial de antiveneno identificado como 15AMP01, fue liberado por el INVIMA el 4 de octubre de 2016. Esto marcó un hito en la producción de antivenenos en Colombia, ya que se desarrolló un tratamiento terapéutico polivalente para tratar envenenamientos por mordeduras de serpientes coral (género Micrurus), incluyendo las cuatro especies de mayor importancia médica: Micrurus dumerilii (mónadas), M. isozonus, M. surinamensis (triadas) y M. mipartitus (bicolor), así como M. helleri, M. medemi, M. sangilensis y M. obscurus mediante neutralización cruzada [12]. El INS ha mantenido una producción permanente de PAA desde el 2016, superando los 2.000 viales por año. Además, realiza el seguimiento farmacológico activo en los pacientes que reciben este antiofídico como terapia para neutralizar los envenenamientos causados por serpientes del género Micrurus (Figura 1).
.png)
Figura 1. Producción histórica de antivenenos del Instituto Nacional de Salud de Colombia
A pesar de los enormes esfuerzos realizados por el INS para garantizar la producción de antiofídicos, las resoluciones 1300 y 1301 del 14 de abril de 2014 declararon una emergencia sanitaria para evitar la escasez de SAP. En el mismo año, el decreto 1375 del 22 de julio estableció los requisitos sanitarios para la fabricación e importación de sueros antiofídicos y antilonómicos (usados como terapia para el envenenamiento causado por las orugas de las polillas del género Lonomia) durante la declaración de una emergencia nacional en salud pública. Posteriormente, la Resolución 1209 del 21 de abril de 2017 extendió la emergencia sanitaria declarada por las Resoluciones 1300, 1301 y 1302 de 2014, que ya habían sido extendidas por las Resoluciones 1241 (2015) y 1478 (2016), por doce meses.
Finalmente, en diciembre de 2017, la planta de producción del INS fue certificada en buenas prácticas de manufactura, y se autorizó la fabricación de SAP y PAA. Además, el INVIMA autorizó al INS a fabricar lotes piloto de antivenenos laquésico, escorpiónico y lonómico. Debido a la escasez de antiveneno lonómico (ALP), cuyo único productor era el Instituto Butantan en Brasil, el INS desarrolló el ALP y envasó un total de 1.663 viales entre diciembre de 2017 y octubre de 2018.
Desde 2012 hasta el presente, el INS ha producido un total de 182.333 viales de SAP y 7.127 viales de PAA. La producción máxima por tipo de antiofídico se logró en 2022 con 49.931 viales de SAP y 2.068 viales de PAA en 2019, siendo la máxima producción de antivenenos en la vigencia 2022 con un total de 50.525, de los cuales 594 correspondieron a antilonómico (Figura 2). La capacidad máxima de producción de la planta se estima en aproximadamente 100.000 viales con los atributos de calidad del SAP, que tiene la mayor capacidad de neutralización entre todos los antivenenos disponibles en Centro y Suramérica [13].
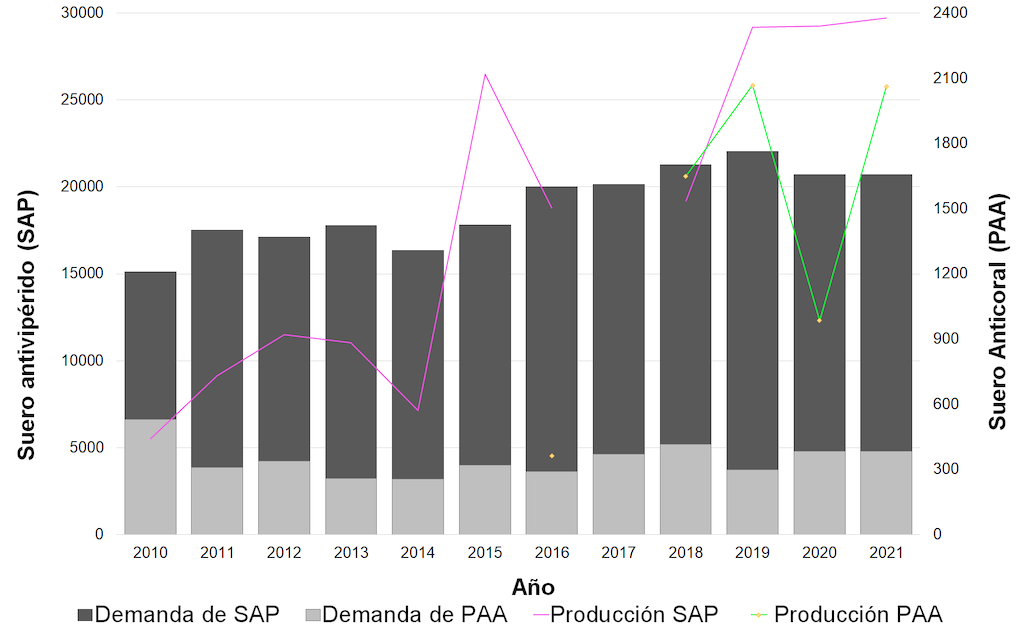
Figura 2. Producción de antivenenos bajo el certificado de Buenas Prácticas de Manufactura 2010-2021. SAP = suero antiofídico polivalente; PAA = antiveneno anticoral polivalente.
La eficacia y calidad del SAP producido por el INS se optimizó desde inicios de la década del 2000, al incorporar a los esquemas de inmunización nuevos venenos de serpientes de importancia médica que abarcan todas las ecorregiones del país. Esto permitió aumentar su versatilidad, así como asegurar que los antiofídicos mantuvieran el mismo antígeno que activa la respuesta inmune del equino. Desde 2011, el INS ha incrementado la representatividad de los venenos de serpientes de varias especies y regiones biogeográficas (ver Capítulo 7 y 8). Esto ha creado un robusto banco de venenos, garantizando su disponibilidad para la producción antiofídicos e investigación. El INS cuenta con un programa de estudio de estabilidad para la producción de antivenenos, en el cual periódicamente se realizan pruebas de control de calidad para verificar letalidad (DL50) de los venenos empleados en la producción, así como evaluaciones de la dosis efectiva media (DE50) todos los antivenenos producidos para asegurar su estabilidad en el tiempo.
La producción de antivenenos del INS cumple con el marco regulatorio vigente y aplicable. Desde 2010, los antivenenos se han fabricado en una nueva planta para sueros hiperinmunes. La planta cuenta con la certificación de Buenas Prácticas de Manufactura (BPM). Esta certificación permite al INS producir sueros antiofídicos polivalentes (SAP), antiveneno anticoral polivalente (PAA) y antiveneno antilonómico polivalente (ALP).
El suero antiofídico polivalente tiene el número de registro sanitario INVIMA 2012M-0013350 desde 2012, otorgado por la Resolución 2012019512 (13 de julio de 2012) y renovado con el número 2019M-0013350-R1 por la Resolución 2019034649 del 12 de agosto de 2019. Es importante destacar que el INS gestiona toda la cadena de producción, desde la colecta de serpientes, la obtención y caracterización de venenos, hasta la obtención del producto terminado, cumpliendo con todos los parámetros de calidad autorizados por INVIMA.
El INS garantiza y asegura la producción de antivenenos mediante el cumplimiento de los protocolos de fabricación y control de calidad. Se verifica el cumplimiento de todas las especificaciones y parámetros de calidad establecidos en cada etapa de la cadena de producción. Además, el control de calidad lleva a cabo un programa de estudio de estabilidad y farmacovigilancia activa. Cada lote fabricado es certificado por INVIMA antes de su comercialización (Tabla 1).
|
Producción histórica
|
Producción de antivenenos
|
|
Periodo
|
Viales de SAP
|
Viales de SAM
|
Viales de PAA
|
|
1975 a 1990
|
120.472
|
20.334
|
0
|
|
1991 a 2011
|
133.869
|
19.898
|
0
|
|
2012 a 2024
|
249.771
|
0
|
9.082
|
SAP: Suero Antiofídico Polivalente, PAA: Antiveneno Anticoral Polivalente, SAM: Suero Antiofídico Monovalente.
Para estimar el número requerido de viales de SAP y PAA producidos por el INS, se considera que la neutralización mínima de toxicidad es de 7 mg/mL para el veneno de Bothrops spp., 1 mg/mL para el veneno de Crotalus durissus, y 0,2 mg/mL para el veneno de M. dumerilii,M. isozonus, M. surinamensis, y M. mipartitus [14]. El número de viales se determinó con base en el criterio establecido por el Ministerio de Salud y Seguridad Social en la «Guía para el Manejo de Emergencias Toxicológicas», y considerando el número de accidentes ofídicos reportados entre 2010 y 2021 en Colombia. La Figura 2 ilustra la producción de suero antiofídico polivalente (SAP) y antiveneno anticoral polivalente (PAA) durante el periodo 2010-2021, evidenciando que la capacidad de producción del INS es suficiente para cubrir las necesidades del país.
1.3 Regulación sobre la producción de antivenenos
El Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) es una entidad gubernamental en Colombia con la misión de proteger la salud individual y colectiva de los colombianos. El INVIMA regula y monitorea el consumo de alimentos, el uso de medicamentos, dispositivos médicos y otros productos que requieren vigilancia sanitaria.
Según las regulaciones del INVIMA para medicamentos y productos biológicos, los antivenenos se clasifican como productos de origen biológico [15]. El INVIMA realiza vigilancia sobre productos biológicos para verificar sus estándares de calidad y seguridad de manera sistemática. El Programa Nacional de Farmacovigilancia del INVIMA tiene por objeto vigilar la seguridad, efectividad y calidad de los medicamentos durante la etapa de comercialización, es decir, luego de obtener la autorización de comercialización por parte del INVIMA. Estas actividades complementan el control y monitoreo de los productos a lo largo de la cadena de producción, minimizando los riesgos e impactos en la salud humana [11].
Se requiere autorización del INVIMA para la comercialización, importación o fabricación de antivenenos. Este permiso se concede solo después de cumplir con todos los requisitos establecidos en las regulaciones sanitarias vigentes, validados por INVIMA [11]. Los principales requisitos incluyen la estandarización y validación de los procesos productivos y métodos de ensayo, la evaluación farmacológica, la certificación de manufactura, el registro sanitario (RS) y el cumplimiento de los estándares de estabilidad de los antivenenos a comercializar. No es posible comercializar un producto biológico en Colombia, como los antivenenos, sin cumplir con cada uno de estos requisitos. Sin embargo, el Ministerio de Salud y Protección Social es responsable de garantizar la salud y seguridad de todos los habitantes del país. Por lo tanto, debe tomar las medidas necesarias para prevenir y controlar cualquier contingencia que pueda afectar a la población, como una posible escasez de antivenenos. En circunstancias excepcionales, como se describió anteriormente, el Ministerio en varias ocasiones ha declarado una emergencia sanitaria nacional debido a la escasez de antivenenos.
En Colombia, hay pocas normas que regulan la producción de antivenenos. El Decreto 821 de 2017 «establece el Reglamento Técnico de Emergencia para la obtención del Registro Sanitario de Antivenenos y adoptó la Guía de Buenas Prácticas de Manufactura para su fabricación». Sin embargo, este decreto fue parcialmente derogado por el Decreto 386 de 2018; este indica que se debe hacer un «dibujo a escala de los borradores de etiquetas y proyectos de los envases y embalajes, que deben incluir: nombre del producto, nombre del fabricante, número de lote, forma farmacéutica, volumen etiquetado, especificidad (veneno neutralizado incluyendo el nombre común de los animales contra los cuales el producto es efectivo), potencia neutralizadora, condiciones de almacenamiento (incluidas las del producto reconstituido cuando se aplique), descripción del proceso de reconstitución, vía de administración, dosis recomendada, contraindicaciones, advertencias y fecha de vencimiento». El anexo técnico del decreto corresponde a la «Guía de Buenas Prácticas de Manufactura (BPM) para la fabricación de antivenenos».
El Decreto 386 de 2018 incluye la descripción del «procedimiento para obtener, renovar o modificar el registro sanitario de antivenenos, e incluye medidas para garantizar su disponibilidad». Países como México y Brasil tienen sus propias farmacopeas, que contienen monografías específicas para cada tipo de antiveneno. Estas monografías direccionan los parámetros de calidad que deben cumplir los diferentes antiofídicos producidos y/o comercializados en cada país. En México, este documento se conoce como «La Farmacopea de los Estados Unidos Mexicanos» y es emitido por la Secretaría de Salud. Para Brasil, se llama «Farmacopeia Brasileira» que es emitida por la Agencia nacional de vigilancia sanitaria - ANVISA.
En estos países es obligatorio cumplir con las disposiciones de cada monografía, que especifica la capacidad neutralizadora mínima que debe tener cada tipo de antiveneno y contra qué especies es efectivo. En Colombia, no hay sueros o venenos de referencia, ni existe una normativa farmacológica que establezca la capacidad neutralizante mínima que deben tener los antiofídicos, ni contra qué especies de veneno se debe llevar a cabo esta prueba. Este vacío legal, técnico y regulatorio debe ser superado para mejorar los procedimientos de obtención del registro sanitario de antivenenos. Estas normas permitirían asegurar que todos los antivenenos disponibles en el mercado colombiano cumplan con la capacidad neutralizante mínima requerida para los envenenamientos causados por especies de importancia médica en el país.
2. Desarrollo y producción de antiofídicos
Los métodos tradicionales para la fabricación de antiofídicos, implican inducir la producción de anticuerpos en un huésped mediante inoculaciones seriadas de veneno de serpiente, considerando su dosis letal media (DL50) [16,17]. El caballo es el animal más comúnmente utilizado debido a su capacidad de producir una gran cantidad de inmunoglobulinas contra el veneno, poseer gran volumen sanguíneo y resistencia [18–20]. No obstante, es bien conocido que los caballos u organismo huéspedes a ser inmunizados, no toleran la inoculación directa del veneno crudo [20]. Por lo tanto, es necesario utilizar adyuvantes durante la aplicación del veneno para formar depósitos del antígeno, y así, promover una liberación que preserve la inmunogenicidad de los toxoides purificados y estimule la respuesta inmune. [21].

Figura 3. Procedimiento de inmunización y producción de plasma hiperinmune. (A) Ordeño de las serpientes para la obtención de veneno. (B-C) Inoculación de veneno de serpiente con adyuvantes en un caballo como modelo animal. (D) Cateterización de la vena yugular para recolección de sangre. (E) Sangre colectada aproximadamente ocho litros. (F) Sedimentación celular luego de 18-24 horas de almacenamiento. (G) Separación mecánica del plasma. (H) Cuatro litros de plasma hiperinmune obtenido luego de la separación. (I) Bolsas con plasma hiperinmune (materia prima para la producción de antiofídicos) y paquete celular reconstituido en solución salina para ser reinfundido a los caballos. (J) Plasma en cuarentena destinado a la planta de procesamiento. Fotos por:(A) Javier Crespo; (B)–(J) Germán Díaz.
El esquema de inmunización varía según la DL50 del veneno [22], la especie utilizada para producir inmunoglobulinas y la técnica de purificación de proteínas [23]. Es importante analizar el título de anticuerpos al final del ciclo de inmunización para evaluar la producción de las inmunoglobulinas deseadas [5,24]. Una vez obtenido el título de anticuerpos deseado, los caballos se someten a sangrados de producción, que permiten la recolección de aproximadamente ocho litros de sangre de cada animal a lo largo de un período de tres a cuatro días, dependiendo de la condición corporal del animal. El plasma se separa mediante sedimentación o centrifugación; los glóbulos rojos se reinfunden por medio de aféresis manual para reducir el riesgo de anemia en los caballos [25,26]. Históricamente, la producción y comercialización de antivenenos a través de formas farmacéuticas ha evolucionado durante cuatro generaciones [27]. La metodología descrita por Pope [28,29] sirve como base para la elaboración de antivenenos.
Los antiofídicos de primera generación consisten en suero no purificado separado de la sangre de animales que han sido hiperimmunizados con venenos [30,31]. Los antivenenos de segunda generación son inmunoglobulinas completas purificadas utilizando técnicas de precipitación con sulfato de amonio o ácido caprílico [32]. La composición de antiofídicos de segunda generación consiste en inmunoglobulinas G (IgG) completas con un peso molecular aproximado de 150 kDa. Es importante destacar que algunas fracciones de otras proteínas, como la albúmina, también pueden estar presentes [33,34].
Los antiofídicos de segunda generación son compuestos de IgG purificadas sin proteínas séricas. Contienen una pequeña cantidad de proteínas de peso molecular medio y alto, que varían entre 1% y 5% [35–37]. Comercialmente, los antivenenos de primera y segunda generación se conocen como sueros, mientras que aquellos compuestos por fracciones F(ab')2 o Fab se denominan faboterapéuticos [38].
Los antivenenos de tercera generación consisten en fracciones de inmunoglobulinas de aproximadamente 100 kDa que se obtienen al digerir las proteínas plasmáticas de IgG completas utilizando pepsina (una proteasa aspártica). Esto separa el fragmento F(ab')2 de las cadenas pesadas y divide el fragmento Fc de las inmunoglobulinas. Luego, el F(ab')2 se purifica mediante un segundo proceso de precipitación con sulfato de amonio [31]. Los fragmentos F(ab')2 impiden que el sitio activo del veneno interactúe con su receptor, neutralizando así los efectos del veneno y promoviendo su eliminación [39]. El tamaño de las moléculas disminuye como resultado de la digestión del Fc mediante clivaje proteolítico, el cual hidroliza más del 50% de las regiones constantes de IgG [40]. El tipo de enzima utilizada y la termocoagulación de IgG pueden afectar el rendimiento de este proceso [23,26]. Métodos adicionales para eliminar lipoproteínas y purificar F(ab')2 incluyen la cromatografía de intercambio iónico y los procesos de precipitación con ácido caprílico. Estos métodos a veces se emplean en la purificación de F(ab')2 [25].
Los antivenenos de cuarta generación son inmunoglobulinas que han sido digeridas por papaína a pH neutro [41,42]. Sin embargo, para cortar los anticuerpos en la región bisagra y romper los enlaces disulfuro, se requiere la incubación con un agente reductor (reducción leve con mercaptoetilamina) [43], lo que resulta en la generación de dos fragmentos Fab y un fragmento Fc [44]. El fragmento Fc puede ser eliminado de la molécula de IgG mediante métodos cromatográficos [45], o mediante tratamiento con pepsina, lo que produce fragmentos Fab monovalentes purificados [43,44]. La reducción de los enlaces disulfuro que unen los fragmentos F(ab) los deja como estructuras individuales con sus sitios de reconocimiento intactos. Cada fracción tiene un peso molecular aproximado de 50 kDa, lo que facilita su eliminación del cuerpo del paciente [37].
Dependiendo del proceso de digestión enzimática o de la técnica de ADN recombinante utilizada, se pueden obtener diferentes conformaciones de los anticuerpos según el propósito [46,47]. Una vez producido el antiofídico, se realiza la primera verificación de inmunogenicidad utilizando técnicas de electroforesis en gel o western blot [48,49]. Un antiofídico con inmunogenicidad positiva es aquel reacciona con todas las fracciones proteicas del veneno [50–54]. Además, la calidad microbiológica y la eficacia del producto deben medirse mediante la técnica de dosis efectiva media (DE50) para determinar su capacidad neutralizante [55–57]. Después de realizar evaluaciones de seguridad y eficacia, el antiveneno se envasa en viales en forma líquida o liofilizada [25,58,59].
Los antiofídicos se clasifican en monoespecíficos o poliespecíficos, dependiendo de si en la inmunización del animal se utiliza el veneno una sola especie de serpiente, o veneno de múltiples especies de serpientes, respectivamente. No obstante, en ambos casos, los venenos son obtenidos de más una población de las especies de serpientes de importancia médica que habitan el territorio o país para el cual fue diseñado el antiofídico [33,60].
La producción de antivenenos monoespecíficos ha sido prácticamente descontinuada a nivel mundial. Sin embargo, algunos fabricantes continúan produciendo antiofídicos monovalentes. Por ejemplo, en Perú, el Centro Nacional de Productos Biológicos produce dos tipos de suero monoespecífico contra mordeduras de serpiente [7]. El suero anticrotálico inyectable se utiliza para tratar envenenamientos causados por Crotalus durissus (serpiente cascabel suramericana), y el suero antilaquésico inyectable para tratar envenenamientos causados por Lachesis muta (matabuey o verrugoso amazónico) [61]. En contraste, la mayoría de los fabricantes producen antiofídicos poliespecíficos que neutralizan el veneno de vipéridos o elápidos, como el antivipérido y anticoral producidos en Colombia por el INS [62,63].
Se han presentado propuestas experimentales utilizando diferentes formatos de anticuerpos para crear nuevos antiofídicos que buscan constituir tratamientos farmacéuticos más eficientes y efectivos [64,65]. Esto se puede lograr no solo mediante la digestión enzimática de inmunoglobulinas, como se mencionó anteriormente, sino también a través de otros métodos. Por ejemplo: (1) mediante la generación anticuerpos monoclonales o recombinantes contra componentes específicos de los venenos [66]; (2) empleando cadenas epítopes sintéticas para la inmunización [67]; (3) mediante la generación antígenos recombinantes a partir del consenso de toxinas específicas que son pobres inmunógenos, pero que pueden enriquecer posteriormente antivenenos existentes, o producir nuevos antivenenos con especificidad exclusiva [68].
Actualmente, existen desarrollos biotecnológicos internacionales en curso que han alcanzado experimentación in vivo e in vitro con resultados prometedores [69,70]. Sin embargo, aún deben ser evaluados estos resultados en ensayos clínicos. Recientemente en Colombia, Romero-Giraldo et al. [71] clonaron y expresaron la fosfolipasa A2 (PLA2) más abundante del veneno de Micrurus dumerilii y generaron IgGs contra la toxina recombinante, los cuales lograron neutralizar las actividades miotóxicas producidas por las PLA2,así como las inducidas por el veneno completo. Si bien estos resultados son promisorios, aún se requieren ensayos clínicos para validar su eficacia y eficiencia.
En Colombia aún es incipiente la implementación de estas tecnologías modernas para la mejora de antiofídicos como tratamiento terapéutico contra los envenenamientos causados por serpientes. No obstante, alentamos a los investigadores a explorar estos senderos para el desarrollo de nuevos antiofídicos.
3. Evaluación preclínica de los antivenenos
Para que un medicamento de origen biológico de uso humano entre estos los antiofídicos, cumpla con los requisitos de calidad, deben someterse a una rigurosa evaluación que permita demostrar su capacidad neutralizante contra las toxinas del veneno, así como su efectividad en el control o reversión de las manifestaciones clínicas causadas por el mismo. La Organización Mundial de la Salud (OMS) propone metodologías para evaluar este tipo de medicamentos. Esto se debe a que la administración parenteral de los antiofídicos derivados de inmunoglobulinas animales es la base para tratar los envenenamientos causados por serpientes, que constituyen un serio problema de salud pública en las regiones tropicales del mundo [72].
Para garantizar la efectividad y seguridad de los antiofídicos para uso humano, es necesario evaluar su capacidad neutralizante y seguridad a nivel preclínico. Esto se debe a la gran variación en la composición del veneno y los tipos de veneno utilizados para la inmunización (ver Capítulos 2, 3, y 5), así como a la heterogeneidad en la eficacia clínica [73]. La evaluación preclínica de los antiofídicos de serpiente proporciona información valiosa para seleccionar los antiofídicos apropiados para su uso en diversos países del mundo [74,75]. La OMS sugiere que los ensayos preclínicos esenciales para determinar la capacidad neutralizante del antiveneno frente a la letalidad inducida por el veneno son: la dosis efectiva media del antiveneno (DE50); y la dosis letal media (DL50), respectivamente. No obstante, dado que los venenos inducen otras actividades tóxicas que también pueden ser evaluadas, a continuación, la Figura 4 presenta los pasos más relevantes para determinación de la eficacia preclínica de un antiofídico durante su proceso de producción.
.jpg)
Figura 4. Proceso de evaluación preclínica de antiofídicos: Ensayos esenciales y suplementarios.
3.1 Determinación de las actividades biológicas de los venenos
Determinación de la dosis letal media
La dosis letal media (DL50) de un veneno se define como la cantidad de veneno que causa la muerte en el 50% de los ratones inoculados. La prueba para determinar la DL50 se realiza de la siguiente manera [76]. Se preparan diferentes dosis de veneno en solución salina y se inyectan por vía intraperitoneal (i.p., máximo 0,5 mL) o intravenosa (i.v., máximo 0,2 mL) en grupos de 4-6 ratones. La OMS [76] recomienda una muestra de cinco ratones (misma cepa y rango de peso definido, generalmente de 18-22 g). Las muertes se registran a las 24 horas (para ensayos con inyecciones intravenosas) o a las 48 horas (cuando se usan inyecciones intraperitoneales). Los ratones de control se inyectan con solución salina. Finalmente, la DL50 se calcula usando métodos estadísticos, como Spearman-Karber, función Probit u otros métodos no paramétricos [77–80].
Determinación de la dosis efectiva media del antiveneno
La dosis media efectiva del antiveneno (DE50) se define como la cantidad de antiveneno, o la relación veneno/antiveneno, que resulta en la supervivencia del 50% de los ratones inyectados con una mezcla de antiveneno y una cantidad letal de veneno [76]. Primero, se debe elegir una dosis de veneno de reto o desafío. La OMS indica que la dosis de reto debe presentar de tres a seis veces la letalidad media (3 a 6 DL50) del veneno previamente evaluado. Luego, la dosis seleccionada de veneno se mezcla con diferentes volúmenes de antiveneno, ajustados a un volumen final constante con solución salina. Las muestras se incuban durante 30 minutos a 37°C. Posteriormente, se inoculan alícuotas por la vía seleccionada (i.v. o i.p.) establecidas en la prueba de reto, y se registra la sobrevivencia según las diluciones preparadas. Se emplean dos grupos de control, el primero se inocula con solución salina (control negativo) y el segundo grupo solo con veneno (control positivo). Finalmente, la DE50 se calcula utilizando los métodos estadísticos descritos anteriormente para estimar la supervivencia del 50% de ratones inoculados. El resultado de la DE50 se presenta comúnmente como la cantidad de mg de veneno neutralizado por mililitro de antiveneno.
Adicionalmente a la evaluación preclínica esencial que determina la capacidad de inhibir la actividad letal de los venenos, los antiofídicos pueden ser sometidos a otros ensayos preclínicos suplementarios que buscan determinar su capacidad neutralizante frente a actividades biológicas específicas de los venenos. Sin embargo, estos ensayos son opcionales y dependen del veneno empleado en la producción del antiofídico, el laboratorio donde es producido, y las leyes particulares de cada país. Por ejemplo, en el marco normativo colombiano, el decreto 386 de 2018, establece realizar ensayos específicos (estudios preclínicos) que soporten técnicamente la capacidad neutralizante contra el veneno involucrado. El INS en cumplimiento de la norma, cuenta con pruebas biológicas estandarizadas y validadas para soportar los ensayos preclínicos esenciales con las pruebas de DL50 y DE50, así como pruebas preclínicas suplementarias de eficacia de los antiofídicos frente a otras actividades biológicas de los venenos como miotoxicidad, hemorragia, necrosis dérmica, actividad coagulante y edematizante, entre otras (Figura 4).
Determinación de la dosis mínima hemorrágica y dosis media de neutralización
La dosis mínima hemorrágica (DMH) se define como la cantidad de veneno capaz de inducir una lesión hemorrágica con un diámetro de 10 mm. Para determinar esto, se preparan diferentes dosis de veneno en solución salina con un volumen final de inyección de 50 µL. Luego, se inyecta intradérmicamente en la piel ventral de ratones rasurados. Finalmente, después de 2-3 horas, los ratones se eutanasian de manera ética, se diseca la piel y se mide el diámetro de la lesión hemorrágica resultante. Se recomienda usar cinco ratones por dosis, mientras que el grupo de control debe inyectarse con solución salina.
El ensayo de neutralización se realiza usando una dosis de desafío de 1-5 DMH a la que se adicionan diferentes dosis de antiofídico. Las muestras se incuban durante 30 minutos a 37°C. Luego, se inocula alícuotas de 50 µL intradérmicamente y los ratones se eutanasian de manera ética 2-3 horas después. La piel se diseca y se mide el diámetro de la lesión hemorrágica. El grupo de control experimental se inocula con veneno. La dosis efectiva media para DMH (DMH50) se define como el volumen de antiveneno, en microlitros, o la relación veneno/antiofídico, que reduce el diámetro de las lesiones hemorrágicas en un 50% en comparación con el diámetro de la lesión en los animales inyectados con la mezcla de veneno/salina de control [76].
Determinación de la dosis mínima necrotizante y dosis media de neutralización
La dosis mínima necrotizante (DMN) de un veneno se define como la menor cantidad de veneno que, cuando se inocula intradérmicamente en ratones, induce lesiones necróticas de 5 mm de diámetro tres días después. El método utilizado para determinar la DMN es el mismo que para la DMH, excepto que la piel se examina tres días después de la inoculación del veneno.
El ensayo de neutralización se realiza usando una dosis de 1-2 DMN, que se mezcla con diferentes dosis de antiofídico. Las muestras se incuban durante 30 minutos a 37°C. Luego, se inocula alícuotas de 50 µL intradérmicamente y se mide el diámetro de las lesiones necróticas resultantes tres días después de la inyección. La dosis efectiva media para DMN (DMN50) se define como el volumen de antiofídico, en microlitros, o la relación veneno/antiofídico, que reduce el diámetro de las lesiones necróticas en un 50% en comparación con el diámetro de la lesión en los ratones inyectados con la mezcla de veneno/salina de control [76].
Determinación de la dosis mínima coagulante (procoagulante) y dosis de neutralización
La dosis mínima coagulante (DMC) se puede calcular utilizando una solución de fibrinógeno bovino (2,0 g/L) (DMC-F) o una solución citratada estándar de plasma humano (DMC-P). La DMC se define como la menor cantidad de veneno que coagula una solución de fibrinógeno bovino (2,0 g/L) en 60 segundos a 37°C (DMC-F) y/o una solución citratada estándar de plasma humano (contenido de fibrinógeno 2,8 g/L) bajo las mismas condiciones (DMC-P). Para medir la DMC, se añaden 50 µL de veneno (que varían de 240 a 0,5 mg/L) a 0,2 mL de fibrinógeno bovino o plasma humano citratado estándar en tubos de coagulación de vidrio a 37°C. Se mezclan las soluciones y se registra el tiempo de coagulación en 60 segundos.
El ensayo de neutralización consiste en mezclar una dosis de MCD-P o MCD-F con diferentes dosis de antiofídico. Luego, las muestras se incuban durante 30 minutos a 37°C y se registra el tiempo de coagulación. Se estima el volumen mínimo de antiofídico, o la relación veneno/antiofídico, necesario para prevenir la coagulación completamente, conocido como la dosis efectiva MCD-F (MCD-F100) o la dosis efectiva MCD-P (MCD-P100) en 30 minutos [76].
Determinación de la dosis mínima miotóxica y dosis media de neutralización
La dosis mínima miotóxica (DMM) se define como la cantidad de veneno que induce un aumento en la actividad de creatina quinasa (CK) en suero o plasma, equivalente a cuatro veces la actividad en suero o plasma de animales control inyectados solo con solución salina. Para medir la DMM, se inyectan 50 µL de veneno en el músculo gastrocnemio derecho. Después de 3 horas, se toman muestras de sangre mediante un corte en la cola y se determina la actividad de CK en suero o plasma utilizando kits de diagnóstico comerciales.
El ensayo de neutralización se realiza retando una dosis de 3 DMM con diferentes dosis de antiveneno. Las muestras se incuban durante 30 minutos a 37°C y se inoculan en el músculo gastrocnemio derecho. Después de otras 3 horas, se toman muestras de sangre mediante un corte en la cola y se determina la actividad de CK en suero o plasma. La dosis efectiva mediana de DMM (DMM50) se estima determinando el volumen de antiofídico en microlitros, o la relación veneno/antiofídico, que reduce la actividad de CK en suero o plasma en un 50% en comparación con la actividad de los animales control inyectados con veneno incubado solo con solución salina [76].
3.2 Ensayos de neutralización
La evaluación de la efectividad del antiofídico es crucial para el tratamiento de los accidentes ofídicos. La Organización Mundial de la Salud [76] especifica que cada lote de antiofídico debe ser probado para determinar su capacidad de neutralizar el efecto letal de los venenos relevantes, utilizando un método de prueba aprobado por la autoridad nacional de control del país donde se fabrica el antiofídico [81]. La investigación sobre antiofídicos tiene como objetivo mejorar el tratamiento de los envenenamientos causados por serpientes mediante ensayos de letalidad. Como se describió anteriormente, estos ensayos incluyen el DL50, que cuantifica la letalidad por veneno, y el DE50, que estima el potencial neutralizante in vivo de un antiofídico [81,82].
No obstante, existen varias técnicas in vitro que se han utilizado para cuantificar la letalidad del veneno, así como la capacidad neutralizante de su correspondiente antiveneno, las cuales incluyen pruebas inmunoquímicas que evalúan la capacidad inmunológica resultante de la interacción entre los antiofídicos y los venenos [57,82,83]. Métodos inmunoquímicos, como el ensayo inmunoadsorción ligado a enzimas (ELISA), pueden combinarse con métodos de electroforesis, como el western blot, y estudios de antivenómica, para proporcionar una detección específica. Las técnicas proteómicas pueden utilizarse para identificar las proteínas del veneno y sus epítopos reconocidos por un antiofídico [86]. Estas técnicas también permiten cuantificar el reconocimiento de un anticuerpo hacia uno o varios antígenos y minimizar la experimentación con animales [83,87,88].
Ensayos ELISA
El principio de esta técnica se basa en la unión de antígenos o anticuerpos solubles a una fase sólida insoluble, como los pocillos de una placa de microtitulación, que preserva la reactividad de los componentes inmunológicos [59]. Un complejo antígeno-anticuerpo se detecta utilizando un anticuerpo específico conjugado con una enzima, seguido de una etapa de lavado. Luego, se añade un sustrato específico para la enzima utilizada, y la cantidad de hidrólisis (cambio de color medido visual o espectrofotométricamente) es proporcional a la cantidad de antígeno presente en la muestra de prueba [87,88]. El ensayo ELISA es altamente sensible ya que detecta niveles de proteínas tan bajos como 1 a 400 ng/mL. La especificidad del ensayo está determinada por la calidad de los reactivos utilizados [82,89].
Estudios preliminares han demostrado que el sistema ELISA puede servir como una alternativa in vitro al ensayo DE50 en ratones, con una correlación significativa entre el DE50 in vivo y el DE50 in vitro [90]. Por ejemplo, el DE50 de 38 lotes diferentes de antiveneno fue estimado para cuatro venenos de serpientes de importancia médica en África y comparado con el DE50 in vivo [90]. Las placas ELISA fueron recubiertas con los mismos venenos utilizados para las curvas de referencia. Se observó una correlación significativa (p<0,001) entre los métodos in vitro e in vivo cuando los resultados de DE50 de los antisueros se relacionaron con sus densidades ópticas [87].
Para servir como un control positivo, los antídotos de referencia (aquellos con un DE50 establecido en ratones) deben ser probados en cada placa ELISA y mantenidos como alícuotas estériles a 4 °C. Generalmente, las opacidades se desarrollan antes de que se pierda la potencia. Los antiofídicos liofilizados y congelados han demostrado gran estabilidad a largo plazo. Sin embargo, estandarizar y distribuir antiofídicos de referencia para cada veneno es impráctico, lo cual es una desventaja significativa [82].
Western blot
El término western blot (WB) fue acuñado por primera vez por el Dr. W. Neal Burnette [91,92]. Este investigador observó que la electroforesis facilitaba la transferencia de proteínas desde geles SDS-PAGE a membranas de papel de nitrocelulosa y que este método parecía funcionar mejor que el papel químicamente modificado [92].
El proceso de Western blot comienza con la separación electroforética de una mezcla de antígenos utilizando poliacrilamida con dodecil sulfato de sodio (SDS-PAGE). Este método se basa en la electrotransferencia del veneno a una membrana de nitrocelulosa, que finalmente se trata con dos anticuerpos. Uno de estos anticuerpos está acoplado a una enzima que permite visualizar los componentes de interés.
Los componentes unidos a la nitrocelulosa son accesibles para la interacción con anticuerpos u otras moléculas [59]. El primer anticuerpo es el antiveneno que debe unirse a las proteínas del veneno, mientras que el segundo anticuerpo está acoplado a una enzima que degrada un sustrato y produce una señal colorida que indica los componentes reconocidos por los antivenenos [59]. Esta técnica no puede observar el reconocimiento de epítopos estructurales que podrían ser relevantes, ya que las proteínas del veneno en el gel están desnaturalizadas. La técnica ELISA es la más adecuada para este propósito. Sin embargo, la ventaja del Western blot radica en que se realiza a partir de geles SDS-PAGE, que pueden detectar la mayoría de las bandas de proteínas en el veneno y proporcionar información sobre el peso molecular de los antígenos reconocidos [84,93].
3.3 Antivenómica
La existencia de variaciones en la composición del veneno entre distintas poblaciones geográficas, etapas de desarrollo, e individuos de la misma especie muestra la importancia de llevar a cabo investigaciones proteómicas, toxicológicas e inmunológicas tanto dentro de las poblaciones como entre ellas (ver Capítulos 2, 3, y 5). Estos estudios son esenciales para formular una mezcla óptima de veneno y desarrollar un antiofídico eficaz contra las mordeduras de serpiente causadas por cualquier miembro de la especie [94].
Los ensayos antivenómicos son una herramienta valiosa para evaluar la capacidad de los antiofídicos de reconocer componentes específicos del veneno previamente identificados por espectrometría de masas [52,95]. Esta tecnología utiliza una plataforma centrada en la proteómica, como la antivenómica de inmunorreactividad de los antiofídicos (Figura 5). Proporciona un análisis cuantitativo de las moléculas de anticuerpos terapéuticos y de unión a toxinas presentes en un antiveneno [96]. Este análisis complementa los ensayos de actividad neutralizante del veneno in vivo e in vitro, y podría sustituir a los métodos inmunológicos tradicionales, como ELISA y WB [97].
Antivenómica de primera generación
Estas técnicas se basan en la inmunoprecipitación de toxinas de unión al antiveneno. El veneno completo se incuba con el antiveneno, seguido de la adición de un anticuerpo secundario, como anti-IgG de caballo [86,98]. Los complejos antígeno-anticuerpo inmunoprecipitados contienen aquellas toxinas reconocidas con suficiente afinidad por los anticuerpos del antiveneno. Las toxinas no inmunoprecipitadas son los componentes del veneno que permanecen en el sobrenadante. Estas toxinas representan las proteínas que no son reconocidas, o bien no generaron anticuerpos en el antiveneno, o bien sólo desencadenaron la producción de anticuerpos con muy baja afinidad [99]. Para identificar estos componentes, basta con comparar los cromatogramas de fase reversa de la fracción no inmunoprecipitada con el veneno previamente caracterizado por venómica [99].
Antivenómica de segunda generación
Existen valoraciones de antivenenos basadas en F(ab')2 y Fab, que no pueden evaluarse mediante inmunoprecipitación [98]. Se rellena una columna de cromatografía de afinidad con Sepharose y se añade un tampón de acoplamiento. Tras un periodo de incubación, las moléculas de IgG, F(ab')2, Fab', o Fab de un antiveneno se unirán covalentemente [100]. Luego, la matriz se incuba con una cantidad de veneno fija, las fracciones que contienen las moléculas no retenidas y las que contienen las proteínas que quedaron retenidas en la columna debido a su afinidad con las moléculas del antiveneno se analizan por separado mediante cromatografía de fase reversa [100]. Los antiofídicos usan un enfoque de cromatografía de afinidad para comparar las fracciones retenidas y no retenidas del veneno completo, con el fin de cuantificar el grado en que un antiveneno reconoce las proteínas individuales del veneno [97,101].
La fracción de proteína no unida a la matriz de anticuerpos inmovilizados «i» se estima utilizando la Ecuación 1 [101].
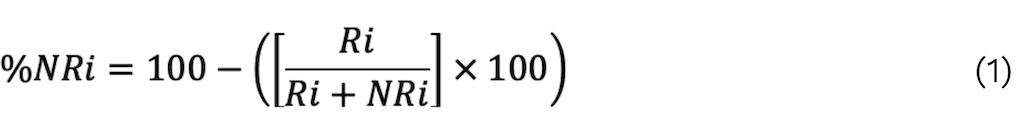
El área del pico cromatográfico de la proteína «i» en la fracción retenida y eludida de la columna de afinidad se presenta mediante «Ri». Este valor se utiliza para determinar la «capacidad antivenómica».
Un valor de %NRi de ≥ 25% indica un buen resultado en las pruebas de neutralización in vivo [8].
Antivenómica de tercera generación
La antivenómica de tercera generación se basa en el mismo principio operativo que la segunda generación, que implica la cromatografía de afinidad en una matriz de antiveneno inmovilizada. Sin embargo, en lugar de utilizar una única concentración de veneno incubada en una única columna de concentración de antiveneno incubada en la matriz de antiveneno, se utiliza una serie de columnas de afinidad idénticas (Figura 5). Cada columna se incuba con cantidades crecientes de veneno. Esta modificación permite determinar la capacidad máxima de unión de cada toxina del veneno al antiofídico inmovilizado [95,102].
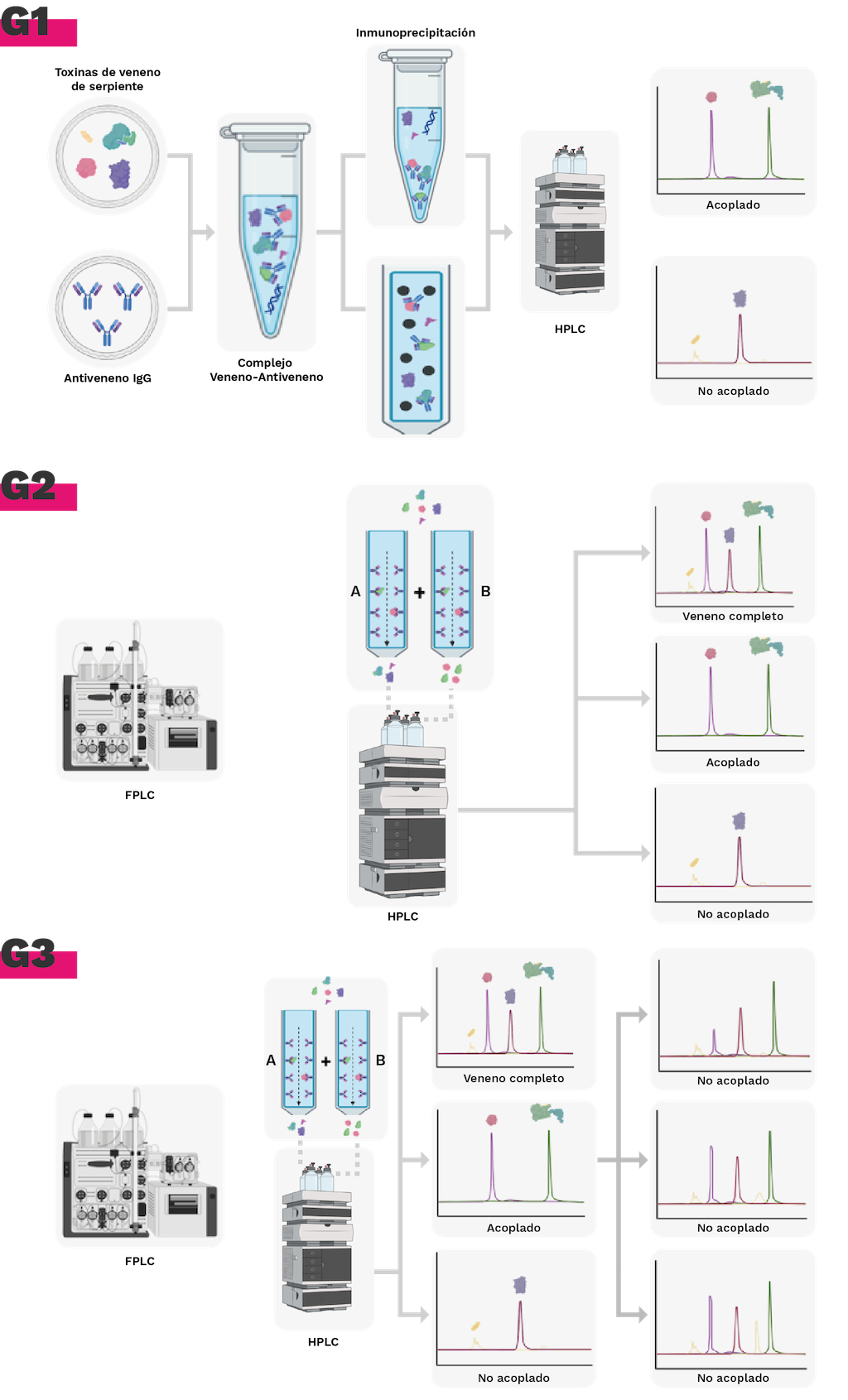
Figura 5. Evolución de la metodología de antivenómica de inmunoreactividad. G1. Antivenómica de 1° generación, inmunoprecipitación de toxinas. G2. Antivenómica de 2° generación, columna de inmunoafinidad con las moléculas de antiveneno. G3. Antivenómica de 3° generación, determinación de capacidad de unión y cuantificación de fracciones con inmunoafinidad. Elaborada en BioRender.
Sin embargo, la antivenómica sólo puede proponer razones potenciales para la reactividad cruzada observada, como la existencia de subfamilias idénticas de toxinas cruciales en dos venenos, uno de los cuales se emplea en la fabricación de antivenenos. La antivenómica carece de capacidad para dilucidar la reactividad cruzada a nivel de epítopo [98].
A pesar de ello, los antivenenos son la única terapia científicamente validada para las intoxicaciones por mordeduras de serpiente. Un aspecto urgente que requiere atención en las instituciones que producen antivenenos es la aplicación de Buenas Prácticas de Manufactura (BPM) para garantizar la sostenibilidad de los proyectos de producción y la disponibilidad y accesibilidad de antivenenos seguros y eficaces. Así como la implementación y ensayo de nuevas tecnologías como la producción de nanocuerpos.
4. Retos y acciones en el tratamiento de mordeduras de serpientes
A diferencia de otras amenazas para la salud pública, el envenenamiento causado por las mordeduras de serpiente no es susceptible de erradicación debido a la inevitable coexistencia entre humanos y serpientes, particularmente en regiones tropicales, y al incremento en los encuentros con estos reptiles como consecuencia de la expansión de los territorios humanos. A pesar de esta realidad, los esfuerzos orientados al desarrollo de alternativas terapéuticas para el manejo del envenenamiento ofídico siguen en marcha, con un enfoque en la optimización y modernización de los procesos de producción de antivenenos.
La implementación progresiva de normativas de bienestar animal en diversas regiones del mundo plantea desafíos significativos, entre ellos la potencial disminución del número de países con capacidad para producir antivenenos a gran escala. En consecuencia, resulta imperativo revisar y actualizar los protocolos de ensayos clínicos que emplean modelos animales, tomando en consideración la relación sufrimiento/beneficio inherente a los métodos actuales. Asimismo, es crucial fomentar el desarrollo y la validación de plataformas alternativas, como ensayos in vitro e in silico, diseñadas para generar resultados sólidos, reproducibles y clínicamente relevantes. Estas herramientas no solo facilitarán la transición hacia un paradigma más ético en la investigación y producción de antivenenos, sino que también garantizarán la sostenibilidad de su fabricación y el acceso universal a este insumo biológico estratégico para la salud pública.
Finalmente, aunque los antivenenos siguen siendo la terapia estandarizada de referencia para el tratamiento de los envenenamientos por mordedura de serpiente, es necesario que la producción de estos se oriente a la generación de antivenenos ampliamente específicos, asequibles, seguros y eficaces. Esto contribuiría a lograr una mayor homogeneidad de los antivenenos en todas las regiones, reduciendo el coste de producción y estabilizando el mercado de estas biomoléculas. Para ello, es fundamental contar con buenos inmunógenos, elegir correctamente el huésped de producción, mejorar los métodos de purificación de anticuerpos, realizar pruebas de antivenenos, incluidos modelos animales alternativos, ensayos in vitro e in silico, así como designar cadenas y sistemas de distribución apropiados para asegurarse que los antivenenos estén disponibles en cualquier localización, especialmente aquellas más cercanas y propensas a una mordedura de serpiente.
