1. Conceptos básicos sobre los venenos de las serpientes
1.1 Sistemas de inoculación del veneno
La capacidad de las serpientes de constreñir e inocular toxinas a sus presas, son tal vez, dos de los rasgos biológicos más conocidos y sorprendentes de estos reptiles. En particular, los sistemas de inoculación de veneno han recibido gran atención debido a su sofisticada especialización, rutas evolutivas y convergencias ecológicas [1–3]. Los mecanismos de inoculación de toxinas se han estudiado principalmente en familias altamente venenosas como Elapidae (p. ej., corales, cobras) y Viperidae (p. ej., víboras, serpiente cascabel), y más recientemente en la familia Colubridae (p. ej., corredoras, cazadoras, bejuquillos, etc.), la familia de serpientes con el mayor número de especies del mundo [1].
El aparato venenoso de las serpientes de las familias Viperidae, Elapidae y Colubridae, cuenta con cuatro unidades o elementos básicos para su funcionamiento eficiente. Primero, el veneno debe producirse y almacenarse en un depósito (=glándula). Segundo, este debe ser transportado al lugar previsto para su inoculación. Tercero, se requiere de un mecanismo para liberar el veneno hacia un organismo objetivo, minimizando el riesgo de pérdida. Cuarto, requiere un mecanismo de propulsión que rodee el reservorio (=glándula), que permita el flujo de veneno a través del sistema, desde el sitio de almacenamiento inicial hasta el organismo objetivo [4].
Específicamente, las glándulas venenosas y las glándulas accesorias, los conductos conectores, los colmillos y los músculos estriados que rodean las glándulas, representan el aparato venenoso en su totalidad. Los primeros tres componentes se presentan en pares, uno a cada lado de la cabeza de la serpiente ubicados en la región temporal y terminando en la región maxilar. Las fibras musculares cubren un área ligeramente más amplia de la cabeza de la serpiente y forman parte del sistema de compresión que permite liberar el veneno. Los mecanismos para la mordedura y propulsión del veneno están compuestos por músculos digástrico, pterigoideo, temporal anterior y temporal posterior, que se encuentran a cada lado de la cabeza [5].

Figura 23. Representatividad de toxinas en los proteomas de venenos de serpientes corales colombianas con datos disponibles. PLA2: fosfolipasa A2; SVSP: serinoproteasas de veneno de serpiente; SVMP: metaloproteasas de veneno de serpiente; LAAO: L-aminoácido oxidasa; CRISP: proteína secretora rica en cisteína; CTL: lectinas tipo C y similares a lectinas; 3FTx: toxinas de tres dedos; KUN: péptidos de Kunitz. Los proteomas del veneno de las especies se obtuvieron de [264,285].
Aunque se ha incrementado la caracterización de los proteomas de veneno de serpientes de especies colombianas, son necesarios más estudios. Debido a las bajas tasas de encuentro, hábitats crípticos y alta riqueza de especies, la mayoría de los venenos de las serpientes de importancia médica en Colombia carecen de caracterización proteómica de sus venenos, siendo las corales (Micrurus) las que presentan los mayores vacíos de conocimiento. Conocer las principales toxinas del veneno de cada especie permitirá entender las actividades fisiopatológicas de cada componente y sus potenciales aplicaciones en biotecnología y medicina.
Potenciales usos de los venenos de serpientes
Los venenos de serpientes son mezclas complejas que contienen toxinas que atacan varios procesos fisiológicos. Han sido reconocidos como fuentes de moléculas bioactivas desde el descubrimiento y desarrollo del captopril (ver Capítulo 10). Ha habido casos exitosos en los que se han producido medicamentos a partir de toxinas de serpientes. Por ejemplo, el tirofibán es un medicamento utilizado para inhibir la agregación plaquetaria en síndromes coronarios agudos. La desintegrina identificada en el veneno de Echis carinatus fue modificada y formulada para desarrollar este medicamento [298].
Los venenos de serpientes también son útiles para estudiar procesos biológicos. Por ejemplo, la batroxobina se utiliza con fines diagnósticos en química clínica. La reptilasa (Reptilase®, Pentapharm, Basilea, Suiza) es una alternativa al tiempo de trombina en muestras que contienen heparina o para diagnosticar hipofibrinogenemia [299]. Los venenos son fuentes importantes de compuestos bioactivos que tienen potencial como compuestos principales para el desarrollo de fármacos o como moléculas para estudiar algunos procesos biológicos (ver Capítulo 10).
Apéndice: Materiales y métodos
Visualización de estructura secundaria de toxinas en 3D
En este capítulo el estudio de las toxinas se aborda desde la estructura secundaria. Para su correcta visualización se colorean selectivamente las zonas con estructura secundaria en hélice alfa (en fucsia), hoja beta (en verde/amarillo), giros y estructura al azar (en blanco).
Se escogió el modo de visualización esquemático para permitir mostrar la representación de Richardson (MolScript). Esta visualización es similar al modelo de cintas, pero muestra mediante puntas de flecha la orientación de las cadenas en hebras y hélices, y los tramos sin estructura secundaria son cordones en lugar de cintas [301,302]. En algunas proteínas, o en ciertas regiones de estas, no existen interacciones de suficiente consideración como para que se pueda distinguir un nivel de organización superior a la estructura primaria. En estos casos se habla de conformación al azar.
Cuando la cadena principal o esqueleto de un polipéptido se pliega en el espacio en forma de helicoide dextrógiro se adopta una conformación denominada hélice alfa. Por lo contrario, cuando la cadena principal de un polipéptido se estira al máximo permitiendo que sus enlaces covalentes interaccionen, este adopta una configuración espacial denominada estructura b (hoja beta), que suele representarse como una flecha [302]. Cuando las estructuras beta tienen el mismo sentido, la hoja b resultante es paralela, y si las estructuras b tienen sentidos opuestos, la hoja plegada resultante es antiparalela.
Las secuencias de la cadena polipeptídica con estructura alfa o beta a menudo están conectadas entre sí por medio de los llamados giros beta, que son secuencias cortas, con una conformación característica que impone un brusco giro de 180 grados a la cadena principal de un polipéptido. Los modelos 3D de las toxinas fueron obtenidos de la base de datos Protein Data Bank (PDB), fueron descargados en formato Wavefront(.obj) y posteriormente optimizados utilizando Blender [303,304]. Posteriormente fueron importados a Jmol generando el patrón de estilo esquemático, luego se exportaron en formato 3D VRML [305]. Finalmente se importaron a la plataforma sketchfab para generar el código html de visualización [306].
Modelos tridimensionales tomados de PDB y preparados en UCSF Chimera v.1.17.3. Todas estas toxinas tienen una vista de modelo 3D disponible en https://serpientes.ins.gov.co/
Recientemente, algunos autores propusieron una guía para separar y estudiar sistemáticamente las toxinas de tres dedos debido a la alta variabilidad entre las secuencias de aminoácidos de estas proteínas [223–225]. Reunieron una base de datos con secuencias 3FTx seleccionadas conocidas para especies de Micrurus de Brasil y clasificaron las proteínas en función de su similitud e identidad estructural. En consecuencia, los autores establecieron parámetros para proponer una reclasificación basada en aminoácidos funcionales clave, puentes disulfuro, distribución de carga en la superficie y la relación filogenética de la secuencia [223–225]. La funcionalidad de la proteína se puede atribuir a nueve grupos con base en la homología de sus secuencias. Esta clasificación sirve como referencia para las toxinas de tres dedos encontradas en especies de Micrurus de Suramérica únicamente.
Los modelos predichos a partir de las secuencias primarias de especímenes brasileños se basaron en secuencias homólogas de elápidos del viejo mundo, incluidos Dendroaspis polylepis, Naja atra, Bungarus multicinctus, B. candidus, Ophiophagus hannah, Dendroaspis jamesoni y Hemachatus haemachatus (cobra escupidora de cuello anillado). Solo se construyó una secuencia a partir de la fulditoxina del veneno de Micrurus fulvius (código PDB 4RUD). Los nueve grupos recientemente conformados incluyeron especímenes locales representativos como Micrurus altirostris, Micrurus carvalhoi, Micrurus corallinus, Micrurus frontalis, Micrurus lemniscatus, Micrurus paraensis, Micrurus spixii y Micrurus surinamensis [224].
Solo se ha purificado y caracterizado una pequeña cantidad de toxinas de tres dedos a partir de venenos de Micrurus colombianas. Actualmente, solo tres toxinas tienen modelos tridimensionales predichos a partir de secuencias reportadas en la literatura. Dos de estas toxinas pertenecen a especies de serpientes de importancia médica en Colombia (M. mipartitus y M. dumerilii; Tabla 4) [225]. Además, ejemplifican los grupos de patrones bicolor y de mónadas, respectivamente [235]. Otra especie del grupo de patrones de mónadas, M. clarki, también ha sido objeto de investigación de 3FTx [227].
Tabla 4. Estructuras de toxinas de tres dedos purificadas de algunos venenos de Micrurus en Colombia. A cada secuencia se le asigna un número de acceso en Uniprot
Figura 1. Tipos de arquitectura dental en serpientes. (A) Aglifa (anaconda verde Eunectes murinus, UF 84822). (B) Opistoglifa (serpiente de casa costera Thamnodynastes pallidus, UMMZ 246849). (C) Proteroglifa (serpiente coral Micrurus nigrocinctus, UMMZ 131984). (D) Solenglifa (serpiente de cascabel sudamericana Crotalus durissus, UMMZ 119571). Todas las imágenes fueron obtenidas el 05/08/2023 de Morphosource bajo la licencia CC BY-NC-ND 4.0.
El aparato venenoso de las serpientes se caracteriza por sus colmillos, los cuales siempre se encuentran en la maxila superior y nunca en ningún otro hueso portador de dientes [6]. Las serpientes con colmillos presentan cuatro tipos de arquitectura dental (Figura 1): colmillos frontales cortos, con canales abiertos o parcialmente cerrados que conducen el veneno a través de un surco visible que conecta con los orificios (dentición proteroglifa: Elapidae y Atractaspididae, como las serpiente coral o la serpiente áspid africana, respectivamente; Figura 1C); colmillos frontales largos y tubulares con conductos cerrados que transportan el veneno una superficie lisa entre los orificios (dentición solenoglifa: Viperidae, cascabeles o víboras; Figura 1D); y colmillos acanalados sólidos y agrandados ubicados en el extremo posterior del maxilar (dentición opistoglifa: colmillos traseros de falsas cobras de agua; Figura 1B); estos esta presentan en numerosos linajes de colúbridos (los surcos pueden estar presentes en la región anterior, posterior, lingual o labial del colmillo). La posición frontal de los colmillos en las estructuras de los proteroglifos y solenoglifos difiere no sólo en la estructura de los colmillos, sino también en el alto nivel de libertad cinemática del hueso maxilar. En los solenoglifos, los colmillos pueden ocupar posición casi paralela al paladar cuando las dos estructuras están en reposo y cercanas entre sí, permitiendo un giro de más de 120 grados a lo largo del eje anteroposterior para colocarlos directamente hacia la presa objetivo [4].
El origen evolutivo de los colmillos, así como de los otros elementos del aparato venenoso, ha sido objeto de controversia, ya que su evolución involucra varias fuerzas subyacentes que han impulsado su diversificación [2]. A pesar de las incógnitas evolutivas no resueltas, la mayoría de los estudios coinciden en que los colmillos frontales presentes en los vipéridos y elápidos, derivaron independientemente de las serpientes con colmillos traseros (Colubridae), lo que refleja una evolución convergente [4]. La evolución del sistema de inoculación de veneno se ha asociado frecuentemente con especializaciones en la dieta y ecología de estos reptiles. Por ejemplo, entre las serpientes venenosas, existen dos estrategias de caza predominantes: las serpientes con colmillos traseros y los elápidos suelen exhibir una estrategia de morder y sujetar (constricción), mientras que la mayoría de las vipéridos y Atractaspis utilizan una estrategia de morder y soltar, lo cual parece estar asociado al tamaño de la presa [2,7]. Por lo tanto, el alargamiento del colmillo sugiere que ha sido una adaptación impulsada por el comportamiento de ataque más que la mayor tensión (fuerza de estiramiento) asociada con cambios estructurales en el aparato venenoso [2].
Dado que las serpientes se mueven lentamente y tienen una tasa metabólica baja, el aparato venenoso descarga rápidamente el veneno en la presa para asegurar la presa, o como forma de defensa contra los depredadores, reduciendo así el gasto energético [5]. Hay dos mecanismos generales para la liberación del veneno. El primero, presente en los vipéridos y elápidos, es un sistema de inoculación alta presión en el que se libera rápidamente un flujo de veneno mediante el aumento repentino de presión. El segundo, presente en los colúbridos con colmillos traseros, es un sistema de inoculación de baja presión, donde la liberación del veneno es más prolongada [1]. En este sistema de baja presión, la liberación de veneno es provocada por la fuerza mecánica obtenida cuando los colmillos penetran en la presa. En el sistema de alta presión, la acción directa de los músculos de la mandíbula sobre la glándula del veneno genera una presión que provoca una rápida liberación del veneno. El sistema de alta presión posee un sello hermético entre el conducto y el colmillo, asegurando que se mantenga la presión en todo el canal de conducción e impulsando el veneno hacia la presa. En contraste, en el sistema de baja presión carece de este sello hermético, lo que resulta en una menor presión del flujo de veneno [1].
Las serpientes de la familia Colubridae poseen un aparato «venenoso» diferente (ver Capítulo 4). La glándula de Duvernoy, que es homóloga a la glándula venenosa y está ubicada en una posición similar, carece de un gran lumen en su reservorio, así como de los músculos de compresión a su alrededor de la glándula. Este sistema utiliza presiones relativamente más bajas que el sistema de inoculación presente en los vipéridos y elápidos, liberando las secreciones lentamente en el epitelio bucal adyacente a los colmillos posteriores de la maxila, los cuales puede o no ser acanalados [1,2,7,8]. La estructura de la glándula de Duvernoy varía ampliamente entre las especies de colúbridos, desde estar ausente hasta presentar una glándula puramente serosa y bien diferenciada [3]. De manera similar, las secreciones de esta glándula muestran un grado significativo de variabilidad en su composición, produciendo toxinas de origen enzimático digestivo o pancreático, así como fosfolipasas, fosfodiesterasas y proteasas [3] (ver Capítulo 4).
La investigación inicial sobre los venenos de serpientes se centra en sus propiedades tóxicas y secreciones orales. Es evidente que los componentes de los venenos, incluidos los de las glándulas de Duvernoy, tienen múltiples funciones biológicas e incluso fines biotecnológicos [5] (ver Capítulos 4 y 10). Los sistemas venenosos de elápidos y víboras difieren en tamaño y morfología, pero todos comparten un diseño básico similar de composición y función.
1.2. Definición de venenos
Para definir el veneno con precisión, seguimos la propuesta de Arbuckle et al. [10], quienes definen el veneno como «una sustancia biológica producida por un organismo que contiene moléculas (toxinas) que interfieren con los procesos fisiológicos o bioquímicos en otro organismo. El organismo venenoso ha desarrollado esta sustancia para proporcionarse beneficios (alimentación y/o defensa) al inocularla sobre otro organismo. El veneno se produce y almacena en una estructura especializada y se transfiere activamente a otro organismo a través de una lesión causada por un sistema de inoculación especializado».
En un contexto biológico, el término «tóxico» se refiere a la propiedad letal de una sustancia química, expresada como la dosis letal media (DL50) o la dosis letal absoluta (DL100), generalmente identificada y caracterizada en condiciones de laboratorio definidas. El término «venenoso» se refiere a la función o rol biológico de la secreción de una sustancia producida por un animal que se utiliza para la defensa o la obtención de otro animal como presa [6]. La observación del animal en su hábitat natural suele ser la base para concluir si una secreción se utiliza como veneno. Los dos términos se basan en conceptos diferentes, por lo que hay más en juego que la mera semántica [3].
Los venenos son mezclas de proteínas, enzimas, péptidos, iones, carbohidratos y trazas de otras moléculas, producidas en las glándulas y secretadas a través de sistemas especializados para someter a las presas, digerirlas o disuadir a los depredadores (p. ej., venenos de serpientes, escorpiones o arañas, entre otros). Por el contrario, lo que se denomina como «ponzoña» (para relacionarlo con el término poison, en inglés) está compuesto por sustancias que se concentran en el cuerpo de ciertos organismos o regiones anatómicas de los mismos. Puede causar efectos adversos en otros organismos que los manipulan o consumen (p. ej., espinas de pez león o piel de ranas venenosas que se encuentran en la familia Dendrobatidae). Las toxinas son sustancias producidas por organismos vivos que pueden alterar la homeostasis de otros organismos están expuestos a ellas [10].
Ambos términos involucran la composición de toxinas, y la toxinología es el estudio de estas sustancias. Se trata de una rama de la toxicología que se encarga de estudiar las moléculas producidas por organismos vivos. Estas moléculas se reconocen como venenos cuando son inoculados de forma activa por un animal a otro; y como ponzoña cuando las toxinas albergadas en los tejidos de animales, plantas, hongos y bacterias son ingeridas, absorbidas o inhaladas por un organismo causándole daño [4,11].
Ser venenoso es un rasgo ecológico funcional que se comporta como intermediario en las interacciones entre dos o más organismos. Sin embargo, es importante comprender que el término «venenoso» no necesariamente significa ser «peligroso» (Figura 2). El nivel de peligro depende de la susceptibilidad al veneno por parte del organismo objetivo, así como de la cantidad de veneno que se inyecta. Por ejemplo, en el caso de una mordedura seca (mordedura de serpiente sin inoculación de veneno) [12], es posible que el veneno ni siquiera llegue al organismo. El veneno es un rasgo común entre las serpientes de importancia médica (familias Elapidae y Viperidae) [13]. Sin embargo, para algunas especies «no venenosas», como ciertos colúbridos con colmillos traseros [19] (ver Capítulo 4), sus secreciones causan síntomas leves de envenenamiento, o en casos poco frecuentes coagulopatías que pueden poner en riesgo la vida las personas.
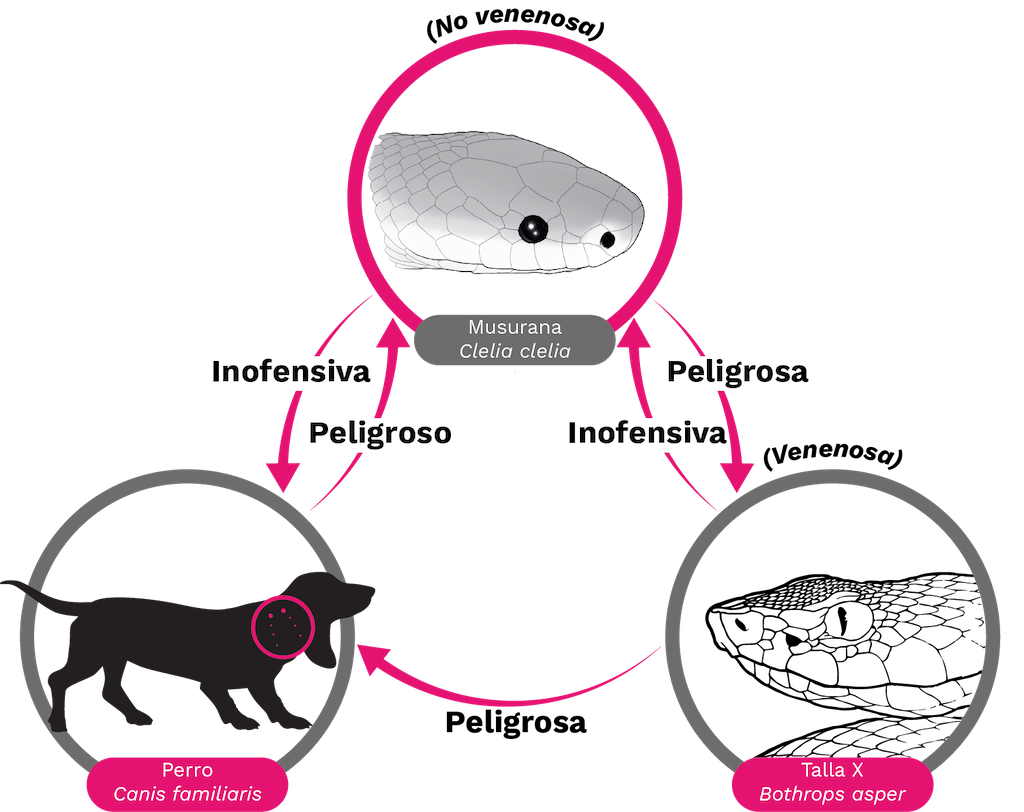
Figura 2. ¿Venenoso = Peligroso? Un toxicólogo clínico describiría a Bothrops asper como venenosa porque su mordedura tiene consecuencias nefastas para los humanos y muchos mamíferos como los perros. Sin embargo, para la serpiente cazadora Clelia clelia (musurana), una B. asper es una comida deliciosa y no venenosa según la definición que requiere la muerte o una actividad farmacológica adversa [20–23].
La composición y actividad de los venenos de serpientes evolucionó en paralelo con la fisiología de sus presas y, posiblemente, de sus depredadores naturales. Aunque anteriormente se creía que los humanos no habían ejercido una presión selectiva significativa en las funciones defensivas de los venenos de las serpientes, y que el envenenamiento se consideraba un daño colateral en la carrera armamentista química entre las serpientes venenosas y sus presas [15], investigaciones recientes sugieren que el veneno y el mecanismo de inoculación de las cobras escupidoras evolucionaron para hacer frente a los homínidos y mamíferos que las amenazaban hace 2,5 millones de años. El veneno de las cobras escupidoras (p. ej., Hemachatus haemachatus, Naja sumatra) evolucionó en parte para producir dolor severo en los homínidos que representaban una posible amenaza para ellas [18]. Por lo tanto, existen múltiples dimensiones de la evolución del veneno de las serpientes que permanecen inexploradas y requieren más investigación.
1.3. Variación entre y dentro de las especies
Los venenos de serpientes muestran una notable variación en su composición y actividad biológica, entre especies y dentro de sus poblaciones, así como en niveles taxonómicos superiores (familias y géneros). Esta variación se considera adaptativa porque permite a las serpientes diversificar su dieta de acuerdo con las presas fácilmente disponibles o disuadir a los depredadores [15]. La variabilidad del veneno tiene implicaciones significativas tanto para la investigación básica del veneno como para el manejo del accidente ofídico, así como en la selección de antiofídicos y especímenes para la producción de antiofídicos que aborden la fisiopatología del accidente [15,19,20].
La variabilidad en los venenos se presenta en múltiples dimensiones (ver Capítulo 3) incluyendo variaciones entre familias, géneros, entre especies e intraespecíficas (entre población o sexos), así como variación entre individual, a través de su desarrollo ontogenético, cambios estacionales, dieta y hábitats [21].
A nivel de familia, los venenos de los elápidos y los vipéridos presentan diferencias significativas. Algunas toxinas son exclusivas de cada linaje, ya que han sido incorporadas de manera única en los venenos de una familia y no están presentes en la otra. [22]. De manera similar, existen diferencias en la composición del veneno entre géneros dentro de cada familia y entre especies dentro de cada género. El enfoque terapéutico debe basarse en el entendimiento de que los antivenenos se han desarrollado con base en la comprensión de la variabilidad de los venenos entre familias, géneros, especies y regiones [15].
Recientemente, se ha reconocido que el veneno varía dentro de las especies, incluidas variaciones regionales, así como variaciones entre edades y tamaños (ver Capítulo 2). El veneno es un rasgo ecológico que evoluciona de forma dinámica. La composición del veneno de una serpiente también está influenciada por la variedad de su dieta. Las serpientes juveniles tienden a consumir presas diferentes a las de los adultos de la misma especie, exhibiendo diferentes estrategias de alimentación y comportamiento. Por ejemplo, los juveniles pueden ser nocturnos mientras que los adultos son más diurnos. Como resultado, los juveniles pueden emplear una estrategia de morder y sujetar, mientras que los adultos pueden morder y soltar [15].
La variación regional puede estar relacionada con diferencias ecológicas entre poblaciones, las cuales están sujetas a mecanismos evolutivos de selección positiva o neutral sobre los sistemas venenosos de las serpientes [23]. Esto sugiere que para que un rasgo evolucione rápidamente, como lo es el veneno, debe haber una diversidad hereditaria significativa dentro de las poblaciones. Por ello, se ha hipotetizado que las variaciones entre los venenos deberían ocurrir con más frecuencia en los miembros adultos de una población, más que en otro segmento poblacional [15].
En general, el marco conceptual molecular sobre el dinamismo de la evolución de los venenos está bien establecido. Por ejemplo, los análisis transcriptómicos y proteómicos del veneno de Bothrops atrox revelan que los mecanismos de transcripción y traducción facilitan la variación fenotípica de un veneno. La evidencia revela que los venenos contienen dos tipos de proteínas: proteínas de función central conservadas, que son esenciales para su funcionamiento adecuado, y proteínas adaptativas, que están menos conservadas, varían en expresión y pueden especializarse en su función [19]. Estas observaciones sugieren que los mecanismos genéticos que controlan la variabilidad del veneno se extienden más allá de la selección de copias de genes o mutaciones en genes estructurales. También incluyen la selección de mecanismos que controlan la expresión genética, contribuyendo a la plasticidad de los fenotipos de serpientes venenosas [19].
La variabilidad del veneno se produce debido a las trayectorias evolutivas de las serpientes venenosas, así como a la especialización de algunas de sus toxinas. Por ejemplo, los venenos tanto de las familias Elapidae y Viperidae están dominados por dos o tres familias de proteínas: fosfolipasas A2 (PLA2) y toxina de tres dedos (3FTx) para los elápidos, y metaloproteasas (SVMP), PLA2 y serino proteasas (SVSP) para los vipéridos. En promedio, estas familias de proteínas constituyen el 83% y el 67% del proteoma del veneno de elápidos y víboras, respectivamente [24].
Los venenos de serpientes de la familia Elapidae son principalmente neurotóxicos, mientras que los de la familia Viperidae inducen coagulopatías, hemorragias y daños tisulares locales [25,26]. Esto se debe a la prevalencia de las proteínas 3FTx y PLA2 en el veneno de elápidos que pueden causar debilidad similar a la miastenia gravis [27]. Esta debilidad puede afectar a cualquier músculo; y si el bloqueo neuromuscular afecta a los músculos de la respiración, puede provocar la muerte [26,27]. Por otro lado, el veneno de vipéridos tiene efectos principalmente hemotóxicos y miotóxicos. Los agentes identificados en el veneno de las víboras incluyen proteinasas similares a la trombina que provocan coagulopatía [28], hialuronidasas que alteran las matrices extracelulares, fosfolipasas A2 que causan inflamación local y dolor [29], y metaloproteasas que contribuyen a la hemorragia [30,31]. El resultado es una destrucción local del tejido empeorada por el efecto coagulopático [26].
Existe una amplia variación en los perfiles de veneno entre especies dentro del mismo género [32–34]. Por ejemplo, los perfiles proteómicos de los venenos de dos especies de vipéridos del género Bothriechis, B. lateralis y B. schlegelli, muestran que los venenos de estas dos especies contienen péptidos potenciadores de bradicinina (BPP) y proteínas PLA2, serinoproteasas, L-aminoácido oxidasas (LAAO), proteínas secretoras ricas en cisteína (CRISP) y metaloproteasas dependientes de Zn2+ (SVMP). Sin embargo, cada especie tiene una abundancia relativa diferente de cada familia de proteínas. Además, cada veneno contiene componentes distintos. Por ejemplo, B. lateralis contiene factor de crecimiento endotelial vascular (VEGF) y moléculas similares a lectina de tipo C, mientras que B. schlegelii tiene inhibidores de proteasa de tipo Kasal [35].
Las proteínas del veneno de ambas especies de Bothriechis son similares en menos del 10%, lo que indica una divergencia significativa en la composición del veneno. A pesar de que ambas especies se adaptan a hábitos arbóreos, las características bioquímicas de su veneno probablemente estén relacionadas con las características de las presas que consumen [32,35]. La composición de los venenos puede proporcionar pistas para racionalizar los diversos signos de envenenamiento causados por B. schlegelii y B. lateralis [35].
También existe variación en el perfil del veneno de serpiente entre individuos de la misma especie, encontrándose diferencias intraespecíficas por ubicación geográfica [28,36,37]. Por ejemplo, se analizó a nivel de subespecie la composición y el perfil toxicológico del veneno de la serpiente cascabel Crotalus simus en México. Los venenos de la subespecie C. s. simus, C. s. culminatus y C. s. tzabcan difieren en la expresión del complejo neurotóxico «crotoxina». Crotalus s. simus tiene la mayor concentración de crotoxina seguida de C. s. tzabcan, mientras que el veneno de C. s. culminatus está casi desprovisto de este PLA2 neurotóxico. El análisis proteómico se correlaciona estrechamente con los perfiles toxicológicos. Por ejemplo, C. s. simus contiene altas cantidades de crotoxina y serinoproteasas, mientras que el veneno C. s. culminatus tiene mayores cantidades de metaloproteasas y crotamina. Esto sugiere que la variación geográfica en la composición del veneno puede reflejar la selección natural para alimentarse de presas locales [38]. La creciente aparición de crotamina en poblaciones de Crotalus es razón suficiente para advertir sobre la necesidad de desarrollar un antiofídico que pueda neutralizar esta toxina [36].
Asimismo, existe variación en la composición del veneno entre especies de serpientes jóvenes y adultas debido a diferencias en la dieta [39]. Normalmente, las víboras jóvenes se alimentan de animales ectotérmicos (invertebrados, anfibios y reptiles), mientras que los adultos se alimentan principalmente de pequeños mamíferos (endotermos) [38,40,41]. Este cambio de dieta ha promovido numerosos estudios que buscan comprender los cambios ontogenéticos en los venenos de las serpientes [42,43]. Por ejemplo, los venenos de Bothrops obtenidos de adultos y recién nacidos revelaron diferentes actividades de coagulación del plasma. La investigación identificó dos perfiles de veneno distintos: los individuos jóvenes tienen pocas miotoxinas PLA2 K-49 y más proteasas, mientras que los adultos mostraron mucha más PLA2 K-49 pero menos proteasas [30,50]. El veneno de los neonatos es menos inflamatorio y hemorrágico, pero más miotóxico y procoagulante en comparación con el veneno de los adultos que muestra mayor actividad hemorrágica in vivo [45–47].
Se ha establecido que ciertos perfiles de veneno podrían variar entre sexos, sugiriendo que esta es una adaptación a la dieta diferencial entre machos y hembras en algunas especies [38,41,48,49]. No obstante, análisis de electroforesis de los venenos de 30 crías de una sola camada de Bitis gabonica (víbora de Gabón) reveló variaciones individuales, pero no se observaron diferencias significativas acorde al sexo [49]. En las serpientes colombianas, las relaciones entre la variabilidad del veneno y los rasgos de historia de vida representan un campo poco explorado que requiere mayores esfuerzos investigativos.
La acción del veneno, así como su combinación proporcional, están determinadas, al menos en parte, por el tipo de presa disponible para una población de serpientes [20,50]. Por ejemplo, es importante señalar que las toxinas CRISP abundan en los venenos de especies de colúbridos como Telescopus dhara (serpiente gato árabe) y Trimorphodon biscutatus (serpiente lira occidental) que se alimentan de reptiles. El efecto hipotérmico tóxico de esta toxina es útil para frenar el movimiento de presas ectotérmicas «de sangre fría» [51]. Los determinantes en la variación de los perfiles de los venenos de serpiente es un campo de investigación apasionante y sin resolver, que requiere un enfoque multidisciplinario, así como un arduo esfuerzo para revelar los mecanismos que generan sus intrincadas mezclas.
2. Lo esencial de los venenos de serpiente
2.1. Metaloproteasas de venenos de serpiente
Las metaloproteasas del veneno de serpiente (SVMP) causan hemorragia en la microvasculatura de sus presas, lo que contribuye a su inmovilización, situación que también se observa en los accidentes ofídicos. Además, los SVMP inducen miotoxicidad, edema, dermonecrosis, formación de ampollas y trastornos de la coagulación [52,53].
La mezcla de veneno contiene varias toxinas, incluidas las SVMP, que pueden descomponer las proteínas en polipéptidos o aminoácidos más pequeños. Las SVMP son un tipo de endopeptidasas dependientes de zinc que pertenecen a la subfamilia ADAM/Adamalisina/reprolisina, que forma parte de la familia de las metzincinas [54,55]. Las metzincinas son metalopeptidasas que tienen dominios catalíticos globulares. Muchos de estos dominios son multidominio, contienen una secuencia consenso de aminoácidos de unión al zinc, HEXXHXXGXX, y un giro β-1-4 que tiene un residuo de metionina altamente conservado (giro Met). Este residuo forma una base hidrofóbica para el cofactor de zinc y los tres residuos de histidina involucrados en la catálisis [56]. Por tanto, estas toxinas están compuestas por cinco dominios o unidades funcionales y/o estructurales. Primero, un corto péptido de señalización (~ 16-32 aminoácidos) que incita a una célula a translocar la proteína, y posteriormente, la SVMP que pueden contener prodominio, metaloproteasa, desintegrina y rico en cisteína [56].
Sin embargo, las toxinas maduras (proteínas funcionales) del veneno, pueden estar en diferentes combinaciones en los últimos tres dominios, lo que permite clasificarlas según su masa molecular y composición de dominios. Las SVMP de tipo P-I tienen una masa molecular entre 20 y 30 kDa y presentan solo un dominio de metaloproteasa. Las SVMP de tipo P-II tienen una masa molecular entre 30 y 60 kDa y contienen dominios de metaloproteasa y desintegrina. Las SVMP de tipo P-III tienen una masa molecular entre 60 y 100 kDa y son ricas en dominios de cisteína además de dominios de metaloproteasa y desintegrina [54,57,58]. La última subclase se determina en función de modificaciones postraduccionales, como el procesamiento proteolítico entre las proteinasas, los dominios de desintegrina (clase P-IIIb), la dimerización (P-IIIc), la adición de proteínas tipo lectinas de tipo C (P-IIId) y los P-III canónicos inicialmente descritos conocidos como P-IIIa [54,58].
La estructura del dominio metaloproteasa, el dominio más característico de las SVMP, está formada por cinco láminas β, cuatro hélices α y una hélice α corta en el N-terminal. Las láminas β1, β2, β3 y β5 son paralelas entre sí, mientras que la lámina β4 es antiparalela a las otras láminas β. El dominio se divide en dos partes (subdominios M y S) por la hendidura de unión del sustrato [65]. Dentro de esta hendidura, el Zn2+ está coordinado por el átomo Nε2 de los residuos de histidina ubicados en las posiciones 142, 152 y 156, así como por moléculas de agua catalíticas.
Debajo del sitio activo se encuentra la cadena lateral de un residuo de metionina altamente conservado que forma una base hidrófoba para el cofactor de zinc y constituye el giro Met, una característica altamente conservada dentro de la familia de las metzincinas [59]. Además, estas enzimas requieren un ion Ca2+ para estabilizar su estructura, y la ubicación de este catión es estructuralmente opuesta a la hendidura de unión del sustrato [58,59]. El sitio activo del dominio metaloproteasa incluye un residuo de glutamato (Glu143) que participa en el inicio del ciclo catalítico al desprotonar el agua catalítica (Figura 3).

Figura 3. Estructura de una SVMP P-I: (A) Muestra la disposición de la estructura secundaria, incluidos Met166 y giro Met (Met-turn). (B. Muestra la posición del ion zinc (esfera gris), su coordinación y los residuos catalíticos. (C) Representa el sitio activo de la enzima. La figura se preparó a partir de la estructura con el código PDB 2W15 usando VMD (Visual molecular dynamics). Figura elaborada por Lina María Preciado Rojo.
Después de desprotonar el agua (cesión de un catión hidrógeno), el grupo hidroxilo resultante actúa como nucleófilo y ataca al carbonilo del enlace peptídico que luego se hidroliza. Posteriormente, se forma un oxianión que se estabiliza mediante el cofactor Zn2+. Finalmente, los electrones dislocados en el oxígeno vuelven a su enlace original y los productos de la reacción se liberan con la ayuda de nuevas moléculas de agua [60].
Las SVMP tienen una hendidura de unión al sustrato que desempeña un papel crucial en la unión y estabilización de este para una mayor reacción catalítica. Además, los residuos catalíticos y la coordinación del zinc también contribuyen a la reacción. La hendidura contiene subsitios, incluidos S1, S2, S3, S1', S2' y S3', que interactúan con las cadenas laterales del sustrato. El sitio activo de la enzima sirve como centro, con S1 a S3 ubicado desde el sitio activo hasta el N-terminal, y S1' a S3' ubicado desde el sitio activo hasta el C-terminal. El enlace peptídico que se va a hidrolizar se encuentra en el centro del sitio activo y el sustrato tiene sitios complementarios de la siguiente manera: los sitios P1 a P3 interactúan con los subsitios S1 a S3 de la enzima y los sitios P1' a P3' interactúan con S1' para subsitios S3' de la enzima. Esta nomenclatura permite una descripción exacta de la interacción entre la enzima y el sustrato o inhibidores [60,61] (Figura 4).
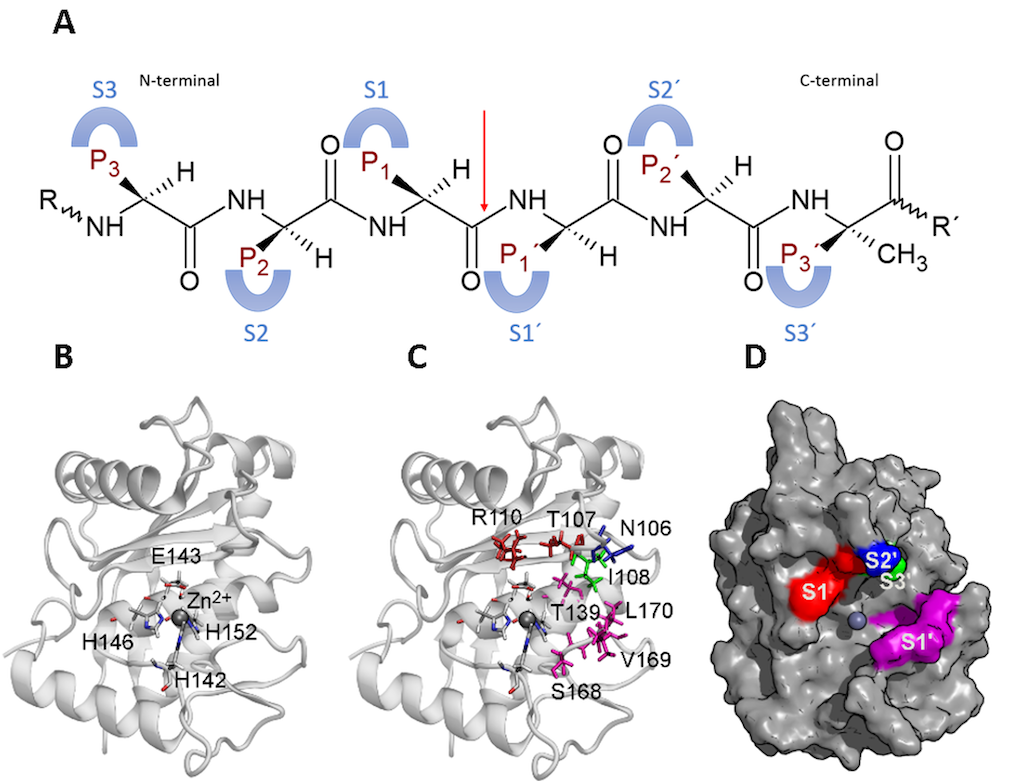
Figura 4. Subsitios de una SVMP P-I. (A) Representaciones esquemáticas de subsitios de la enzima y su interacción con sitios del sustrato. La flecha roja señala el enlace peptídico que se va a hidrolizar. (B) Sitio activo de la enzima y los residuos catalíticos. (C) Cadena lateral de los residuos implicados en los subsitios de la enzima mediante una clave de color. Rojo: S1, azul: S2’, verde claro: S3’ y morado: S1’. (D) Superficie de la enzima y ubicación de los subsitios con la misma clave de color que (C). La figura se preparó a partir de la estructura con código PDB 2W15 usando VMD (Visual molecular dynamics). Figura elaborada por Lina María Preciado Rojo.
2.2. Efectos biológicos inducidos por SVMP
Hemorragia
Esta actividad puede ser local o sistémica y depende del catalizador. Se ha propuesto una hipótesis de dos pasos para explicar cómo estas toxinas inducen hemorragia [52]. Inicialmente, las SVMP hidrolizan el sustrato en la membrana basal (MB), como el colágeno tipo IV, la laminina, el nidógeno y el perlecán. Esto debilita la estabilidad mecánica de la MB y aumenta la distensibilidad de la pared del microvaso. Además, las SVMP pueden degradar otras proteínas, incluidas aquellas implicadas en la unión de la MB a la matriz extracelular de los músculos, como los colágenos no fibrilares VI, XII, XIV, XV, XVI y XIX, así como el colágeno fibrilar tipo V [53,62,63].
En el segundo paso, las fuerzas hemodinámicas actúan sobre la microvasculatura, incluida la tensión de corte y la presión hidrostática sobre la pared del microvaso. Estas fuerzas potencian la distensión de la pared, provocando su ruptura y la extravasación de glóbulos rojos [58] (Figura 5).
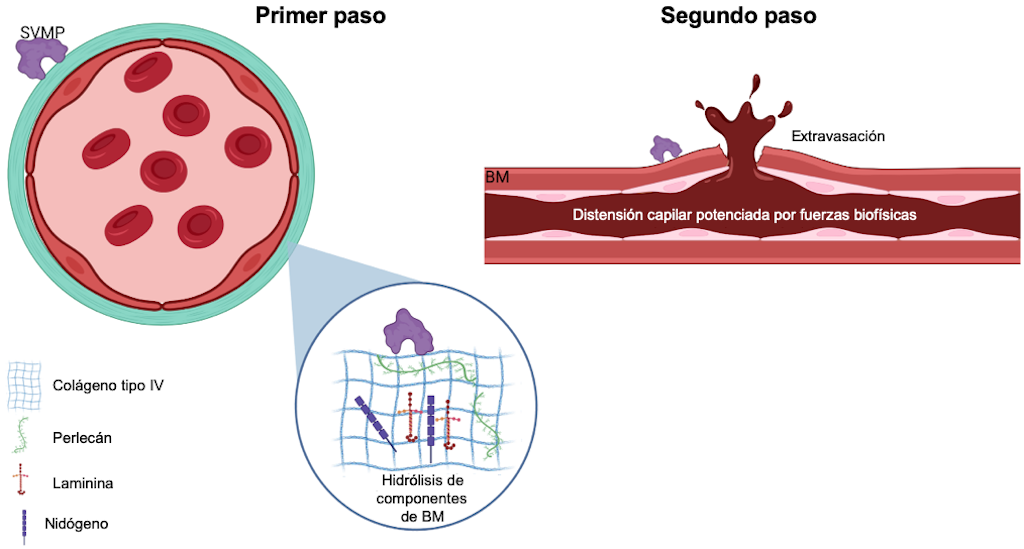
Figura 5. Representación esquemática del modelo de dos pasos para la hemorragia inducida por SVMP. El primer paso implica la hidrólisis de componentes de la membrana basal (MB) que desestabiliza la interacción entre las células endoteliales y la MB. En el segundo paso, las fuerzas biofísicas contribuyen a la potenciación de la distensión capilar de la pared de los microvasos, lo que lleva a su alteración. Para más detalles, consulte el texto. Imagen creada con Bio Render.
Hay dos tipos de hemorragia, por rexis y por diapédesis. La hemorragia por rexis es característica de la microvasculatura capilar. En este caso, la extravasación se produce a través de huecos formados en la pared del vaso, como se describió anteriormente. Además, el grosor de la célula endotelial disminuye, lo que debilita la pared capilar [52,53,64]. Aunque la hemorragia inducida por SVMP es causada principalmente por el modo por rexis, el mecanismo por diapédesis se observa en las vénulas donde los eritrocitos escapan a través de uniones intercelulares ensanchadas en lugar de a través de espacios en las células endoteliales [65].
Además, las SVMP inducen apoptosis (muerte celular programada) de las células endoteliales [66–70]. En ciertos casos, los niveles de proteínas antiapoptóticas de la familia BCL-2 no se ven afectados, mientras que en otros casos los niveles de BCL-XL, otra proteína antiapoptótica, disminuyen [67,70]. Además, se ha observado la activación de las caspasas 3 y 8 [67,70]. Estas proteasas participan en la cascada de señalización intracelular que conduce a la apoptosis celular. Las SVMP pueden aumentar los niveles de expresión de varias metaloproteasas de matriz (MMP) [71], lo que puede aumentar su efecto hemorrágico al degradar la matriz extracelular y una mayor debilidad de la interacción de las células endoteliales derivadas de la médula ósea.
Existe una clara diferencia en la potencia de las SVMP para inducir hemorragia. Específicamente, las SVMP P-III y P-II son más hemorrágicas que las SVMP P-I. El mecanismo molecular subyacente a este fenómeno aún no se ha dilucidado por completo, pero algunas hipótesis proporcionan información sobre este tema. En primer lugar, las SVMP P-II y P-III contienen desintegrina, dominios ricos en cisteína y, en algunos casos, un dominio de lectina de tipo C (P-III). Estos dominios pueden dirigir las toxinas a objetivos específicos en la microvasculatura, concentrándolas en áreas donde pueden causar el mayor daño [58,61].
Por ejemplo, el dominio desintegrina puede dirigirse a las integrinas expresadas en la membrana celular de las células endoteliales [72]. Por lo tanto, las SVMP P-II y P-III muestran un patrón de co-localización con colágeno tipo IV en la pared del vaso. Por el contrario, las SVMP P-I, que carecen de dominios adicionales, están ampliamente distribuidas en la matriz extracelular y no se concentran en los vasos [73,74].
Además, los estudios han demostrado que el inhibidor de la proteinasa plasmática α2-macroglobulina inhibe las actividades proteolíticas y hemorrágicas de varias SVMP P-I, pero no tiene capacidad inhibitoria para las SVMP P-II y P-III [75–77]. Aún se desconoce la base estructural de estas diferencias. Sin embargo, una hipótesis es que los dominios adicionales encontrados en P-II y P-III crean obstáculos para la unión de la α2-macroglobulina, lo que resulta en una falla en el reconocimiento. Este descubrimiento también puede explicar la capacidad de las SVMP P-II y P-III para causar hemorragia sistémica, ya que la α2-macroglobulina no las limita una vez que ingresan a la circulación sistémica, mientras que las SVMP P-I son rápidamente inhibidas por este inhibidor de la proteasa [53].
Otro aspecto importante relacionado con las SVMP es que las de tipo P-I difieren significativamente en su capacidad para inducir hemorragia [78–80]. Aunque tienen actividad proteolítica similar hacia varios sustratos in vitro, algunas de estas enzimas causan hemorragia mientras que otras no [63,79,80].Se observaron diferencias significativas entre los SVMP hemorrágicos y no hemorrágicos en su capacidad para hidrolizar los componentes de la MB in vivo. La toxina BaP1 de P-I SVMP hidroliza el colágeno tipo IV y el perlecán en mayor medida que la SVMP P-I no hemorrágica, llamada leucurulisina-A. El análisis proteómico de los exudados recolectados del tejido muscular inyectado con estas SVMP también reveló diferencias en los tipos de componentes de la matriz extracelular presentes [63].
Sin embargo, los determinantes estructurales detallados de la actividad hemorrágica de los SVMP P-I siguen siendo en gran medida desconocidos. Por lo tanto, la posibilidad de predecir el potencial hemorrágico de los SVMP basándose en el análisis de estructura o secuencia sigue siendo incierta. Sin embargo, una hipótesis reciente sugiere que las diferencias en la dinámica de un bucle ubicado cerca del sitio catalítico pueden explicar la variable actividad hemorrágica [82].
Otro hallazgo importante fueron las diferencias en las superficies electrostáticas entre este tipo de proteínas [83]. Recientemente se han utilizado herramientas bioinformáticas para predecir la interacción de las SVMP P-I hemorrágicas o no hemorrágicas con sus sustratos naturales, como los dominios de colágeno tipo IV, perlecán y laminina [84]. Las SVMP P-I hemorrágicas pueden formar complejos catalíticos con sus sustratos, mientras que las SVMP P-I no hemorrágicas no pueden. Además, las SVMP P-I no hemorrágicas pueden tener un área de volumen mayor de la hendidura de unión al sustrato que las SVMP de P-I hemorrágicas. A pesar de todos los resultados descritos, se necesitan más estudios para aclarar la actividad hemorrágica entre los tipos de SVMP P-I.
Mionecrosis y deterioro de la regeneración muscular
La inoculación de SVMP produce daño muscular necrótico (mionecrosis) causado por una hemorragia inducida por las mismas toxinas. El sangrado afecta el suministro de sangre al tejido afectado, lo que provoca isquemia. La isquemia puede causar hipoxia y daño a las células musculares [30,80,85]. Este efecto puede contribuir a la actividad miotóxica inducida por otras toxinas del veneno de serpiente, principalmente las fosfolipasas A2 (PLA2).
El proceso de regeneración muscular después de una lesión requiere tres condiciones: suministro sanguíneo intacto, restitución de la unión neuromuscular y MB intacta [86]. Además, está implicada una interacción sincronizada entre varias sustancias y células inflamatorias. Las SVMP afectan dos de estos requisitos al degradar los componentes de la MB, lo que resulta en un debilitamiento de la pared de la microvasculatura y la extravasación del contenido de los vasos. La necrosis del músculo liso en la pared de las arterias intramusculares causada por SVMP puede afectar la regeneración muscular al disminuir el suministro de sangre [87,88]. El oxígeno y los nutrientes hacia el tejido afectado disminuyen y el proceso de regeneración muscular se ve afectado [85].
Formación de flictenas y necrosis de la piel (dermonecrosis)
La formación de flictenas inducida por las SVMP es causada por su actividad catalítica contra los componentes de la MB ubicados en la unión dermoepidérmica [71,89,90]. Las SVMP hidrolizan colágeno tipo IV, laminina y nidógeno, entre otras proteínas, en los fluidos de la flictena [89,90]. Después de esta hidrólisis, la epidermis se separa de la dermis, dando como resultado la formación de una ampolla que acumula principalmente fluido plasmático, favorecido por el proceso inflamatorio, así como fibrinógeno y fibrina que son degradados por varias SVMP. La dermonecrosis puede ocurrir cuando las células se desprenden y luego mueren, y posteriormente tiene lugar un proceso regenerativo ineficiente, ya sea con o sin formación de cicatriz [96]. Sin embargo, se necesitan más estudios para obtener más información sobre este tema.
Edema e inflamación
Hipotéticamente, la actividad formadora de edema de las SVMP está relacionada inicialmente con la extravasación del contenido de los vasos sanguíneos al espacio intersticial. Sin embargo, esta hipótesis ha sido refutada parcialmente de forma experimental. Por ejemplo, al inyectar dosis subhemorrágicas de SVMP, se provoca edema, lo que sugiere que este efecto es independiente de la extravasación y es inducido por eventos con múltiples componentes [86]. Primero, se produce la infiltración de leucocitos en el órgano afectado. Luego, ocurre la degranulación de los mastocitos y liberación de histamina que también puede conducir a la activación de los macrófagos [71,91–93]. De manera similar, las SVMP estimulan la liberación de IL-1 e IL-6, lo que conduce a un aumento del nivel de ARNm del factor de necrosis tumoral (TNF), y resulta en la sobreexpresión de esta proteína responsable de los procesos de inflamación [71,93,95].
2.3. Fosfolipasas A2 del veneno de serpiente
Las serpientes venenosas emplean las fosfolipasas A2 (PLA2) para provocar mionecrosis (muerte de células musculares), neurotoxicidad (parálisis de los músculos respiratorios), inflamación, dolor y alteración de la coagulación sanguínea. Todos estos efectos contribuyen a la inmovilización de la presa y pueden observarse en accidentes ofídicos.
Aspectos generales: clasificación, reacción catalítica y estructura general
Existen dieciséis grupos de PLA2 (IA, IB, IIA, IIB, IIC, IID, IIE, IIF, III, V, IX, X, XIA, XIB, XII, XIV) que se clasifican en función de su secuencia, masa molecular, origen, posición de enlaces disulfuro, requerimiento de calcio y otras características [95]. Las PLA2 pueden hidrolizar el enlace éster de la posición sn-2 de los glicerofosfolípidos, produciendo un ácido graso y un lisofosfolípido (Figura 6).
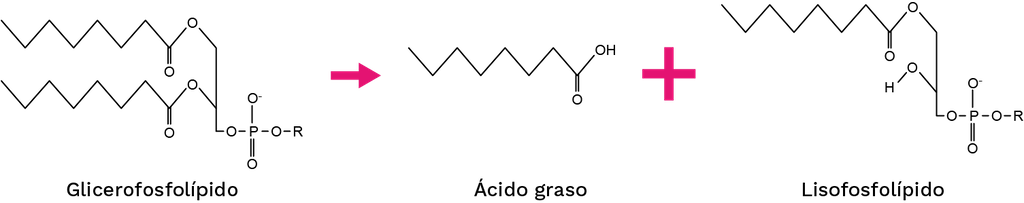
Figura 6. Reacción catalizada por las PLA2s.
La familia de enzimas PLA2 está presente en una amplia variedad de organismos, incluyendo animales, plantas, hongos y bacterias. Sin embargo, entre los dieciséis tipos de PLA2, los encontrados en venenos de serpientes pertenecen a los grupos IA, IIA y IIB [102]. Las PLA2 del grupo IA están presentes en los venenos de los elápidos que en América están representados por las serpientes coral (Micrurus spp.). Estas PLA2 típicamente tienen una masa molecular entre 13 y 15 kDa, y presentan siete puentes disulfuro, uno de los cuales es exclusivo de este grupo y se encuentra entre las cisteínas 11 y 77.
Las PLA2 del grupo IIA se encuentran en los venenos de las víboras, como las serpientes de los géneros Crotalus, Lachesis, Bothrops y Porthidum. La masa molecular de las PLA2 del grupo IIA es como la de las PLA2 del grupo IA, también presentes en los venenos de los vipéridos, ambos grupos con siete puentes disulfuro. El puente disulfuro exclusivo en las PLA2 del grupo IIA se encuentra entre las cisteínas 50 y 137. El grupo IIA comprende otro conjunto de PLA2 de veneno de serpiente, incluida una enzima también aislada del veneno de la víbora de Russell (Daboia russellii) de Asia (India, Pakistán, Nepal, Sri Lanka, Bangladesh y Bután), y la característica única de esta proteína es que tiene un puente disulfuro menos que las PLA2 mencionadas anteriormente [95,96].
Las PLA2 de elápidos y vipéridos tienen contrapartes humanas. Por ejemplo, el grupo IB de PLA2 pancreático es homólogo a las enzimas del grupo IA. La PLA2 sinovial pertenece al grupo IIA y tiene una estructura similar a las proteínas del veneno de serpiente del mismo grupo. Sin embargo, es bien sabido que las PLA2 humanas no causan los efectos nocivos observados en los venenos de serpiente. Estos efectos incluyen neurotoxicidad pre y/o postsináptica, miotoxicidad local y/o sistémica, anticoagulación, cardiotoxicidad, modulación de la agregación plaquetaria, actividad hemolítica, edema e hipotensión, y daño directo a órganos como el riñón, pulmón e hígado [97].
Las PLA2 del veneno de serpiente se clasifican como proteínas básicas o ácidas según su punto isoeléctrico. La mayoría de las PLA2 son básicas. Aunque se sabe que las PLA2 ácidas tienen actividad catalítica, generalmente carecen de efectos biológicos. Sin embargo, en algunos casos, muestran actividades miotóxicas y formadoras de edema [98,99]. Las proteínas ácidas también se han identificado en venenos colombianos, que incluyen especies como Porthidium nasutum, P. lansbergii y Bothrops asper [100–102].
Para explicar la variedad de efectos y las diferencias en la susceptibilidad entre los distintos tejidos, se propuso un modelo que se ocupa de los «sitios diana» en la superficie de las células o los tejidos objetivo [103]. El modelo sugiere que los «sitios farmacológicos» específicos responsables de inducir cada actividad son reconocidos en dichos sitios diana, los cuales se encuentran en la estructura tridimensional de las PLA2. El modelo propone que los sitios farmacológicos pueden o no superponerse con el sitio activo. Los efectos farmacológicos inducidos por las PLA2 están determinados por la alta afinidad entre la molécula diana y el sitio farmacológico. Se supone que esto se debe a la presencia ubicua de fosfolípidos en las membranas celulares. Es poco probable que estas sean las moléculas diana que afirma el modelo. Por el contrario, es plausible que los receptores sean glicoproteínas u otras proteínas que se expresan de forma diferencial [103].
Las PLA2 de los venenos de serpiente son enzimas dependientes de calcio (Ca2+) que estructuralmente consisten en tres hélices α, dos láminas β antiparalelas y un bucle de unión al calcio (Figura 7). Estas proteínas tienen una longitud variable, que va desde 119 a 134 aminoácidos. El canal hidrofóbico involucrado en conducir el sustrato hacia el sitio activo está definido por residuos ubicados en dos de las hélices α (hélice α dos, residuos 37-57, y hélice α tres, residuos 90-109, respectivamente), además de la hélice N-terminal (hélice α uno) y los residuos Leu2, Val3, Phe5, His6, Ile9, Trp19, Val31, Lys69, Ala102 y Ala103. El sitio activo está formado por His48, Asp49, Tyr52 y Asp99. Luego, el bucle de unión al calcio coordina el calcio necesario para la catálisis y está formado por Tyr28, Gly30, Gly32 y Asp49 [104] (Figura 7).

Figura 7. Estructura general de las PLA2 del veneno de serpiente. Las hélices alfa uno, dos y tres se muestran en azul, verde y amarillo, respectivamente. El bucle de unión al calcio se muestra en cian (azul verdoso) y el ion Ca2+ se representa como una esfera azul. Los puentes disulfuro se presentan como barras amarillas. Además, los residuos catalíticos también se muestran en barras (His48, Asp49, Tyr52 y Asp99) (la figura se preparó a partir de la estructura con el código PDB 2QOG, cadena B).
Las PLA2 catalizan reacciones que pueden dar lugar a incoherencia química debido a que las enzimas son solubles en agua, mientras que su sustrato es anfipático (una región de la molécula es hidrosoluble y la otra es liposoluble). Para superar este problema, las PLA2 tienen una superficie de unión interfacial (i-face), que media su adsorción en la interfaz lípido-agua fuera de la membrana celular [104] (Figura 8A).
Para que se produzca una reacción catalítica en la interfase, deben tener lugar una serie de eventos, entre ellos, que el fosfolípido salga de la membrana y llegue al sitio activo de la PLA2. El fosfolípido pasa a través de un canal hidrofóbico mediante un proceso de difusión que no requiere energía. Después de localizar el sustrato en el sitio activo de la enzima, el fosfolípido debe anclarse para reducir los grados de libertad del enlace éster en la posición sn-2. Esta fijación la realiza el aminoácido en la posición 69 (Lys o Tyr) a través de un enlace de hidrógeno, generalmente con el fosfato de la posición sn-3 [104] (Figura 8B).

Figura 8. (A) Representación esquemática de i-face. El círculo negro indica las regiones implicadas en el reconocimiento de la membrana y la adsorción de la enzima en la interfase lípido-agua. (B) Representación esquemática de la conducción del glicerofosfolípido al sitio activo a través del canal hidrofóbico (HC). Las líneas discontinuas azules representan interacciones hidrofóbicas y electrostáticas entre los aminoácidos de la enzima y los glicerofosfolípidos. Los glicerofosfolípidos fueron extraídos de Bio Render.
Tras el reconocimiento del sustrato y su desplazamiento a través del canal hidrofóbico, comienza el ciclo catalítico. La His48 se protona extrayendo un ion hidrógeno del agua, lo que genera un grupo hidroxilo (OH-) que actúa como nucleófilo. A continuación, el OH- ataca el enlace éster sn-2, lo que conduce a la formación de un oxianión que se estabiliza mediante el ion Ca2+. Finalmente, los electrones dislocados del oxígeno vuelven a su enlace original y se liberan los productos de la reacción [104,105].
Las PLA2 del veneno de serpiente requieren actividad catalítica para inducir la mayoría de sus efectos biológicos. Sin embargo, desde 1984 [106] se ha descubierto un subgrupo de estas toxinas que han sustituido su residuo catalítico Asp49 por otros aminoácidos (principalmente Lys, pero también se pueden incluir Gln, Ser y Arg). Esta sustitución hace que las nuevas proteínas sean enzimáticamente inactivas y se las conoce como homólogas de PLA2 o miotoxinas similares a PLA2. Aunque no pueden hidrolizar los glicerofosfolípidos de la membrana, pueden causar miotoxicidad y edema [22,107].
Efectos biológicos inducidos por las PLA 2 s de venenos de serpientes
Miotoxicidad
Las PLA2 del veneno de serpiente pueden inducir mionecrosis a través de miotoxicidad. Aunque los eventos moleculares no se comprenden completamente, existe una descripción detallada de los procesos celulares que involucran el daño de estas toxinas a las células musculares. En primer lugar, se sugiere que la miotoxina se une a la célula diana. Algunos autores han identificado una proteína del músculo de conejo llamada receptores tipo M [108]. El receptor tiene una masa molecular de 180 kDa y contiene repeticiones en tándem de regiones homólogas con los dominios de reconocimiento de carbohidratos (CDR).
Un estudio reciente sugiere que la nucleolina, una proteína nuclear que también se expresa en la membrana celular, puede interactuar con una PLA2 Lys49 y mediar en su internalización [109]. Posteriormente, las PLA2 alteran la membrana muscular. La PLA2 Asp49 cataliza este proceso, mientras que los homólogos de la PLA2 desestabilizan la proteína a través de interacciones hidrofóbicas y electrostáticas con los glicerofosfolípidos.
Fernandes et al. [110] propusieron que estas toxinas tienen un estado oligomérico que puede ser activado alostéricamente por una molécula pequeña, probablemente un ácido graso. La proteína se acopla entonces a la membrana en un sitio específico llamado Sitio de Acoplamiento de Membrana (MDoS). Finalmente, las PLA2 provocan la desestabilización de los glicerofosfolípidos de membrana a través de la acción del Sitio de Disrupción de Membrana (MDiS) [111]. Estos eventos inducen cambios en la selectividad y permeabilidad de la membrana muscular, lo que lleva a una entrada masiva de calcio y despolarización celular. Además, se liberan creatina quinasa (CK), deshidrogenasa láctica (LDH) y otras moléculas que se utilizan como marcadores de miotoxicidad.
La concentración elevada de iones de calcio en el citoplasma de las células musculares conduce a una hipercontracción del sarcómero, lo que resulta en daño mecánico. Los iones de calcio también inducen una sobrecarga de las mitocondrias, lo que lleva a un daño en la cadena transportadora de electrones y la síntesis de ATP. Además, el calcio puede activar las PLA2 citosólicas que pueden aumentar el daño intracelular, particularmente en organelos con membrana; y también puede estimular la acción proteolítica de las calpaínas, desencadenando la desorganización del citoesqueleto. Finalmente, las PLA2 del veneno de serpiente pueden internalizarse, lo que lleva a un mayor daño interno. Estos eventos finalmente resultan en la muerte celular por necrosis (Figura 8), que es un factor significativo en el envenenamiento por vipéridos, incluidos los de Colombia (ver Capítulo 9) [111,112].
La miotoxicidad sistémica es otro efecto biológico conocido de algunas mordeduras de serpientes, observado en Colombia en el caso de accidentes con serpientes de cascabel (Crotalus durissus;ver Capítulo 9) [113]. Aunque los determinantes moleculares de este efecto son actualmente desconocidos, se sugiere que la toxina podría unirse a sitios de baja afinidad en el área inyectada y luego distribuirse a otras regiones anatómicas del cuerpo. Esta distribución puede inducir miotoxicidad a través de los eventos descritos anteriormente [119]. Las PLA2 del veneno de serpiente pueden causar actividad miotóxica que conduce a rabdomiólisis que puede afectar la función renal al bloquear la filtración glomerular debido al exceso de mioglobina en la sangre. Esto en última instancia, puede resultar en insuficiencia renal aguda (IRA) [114,115].

Figura 9. Representación esquemática de los eventos celulares que ocurren durante la miotoxicidad inducida por las PLA2 del veneno de serpiente. TEC: cadena transportadora de electrones; cPLA2: PLA2 citosólica. La membrana de la célula muscular se ve alterada por mecanismos catalíticos o no catalíticos de las PLA2, lo que conduce a una entrada masiva de Ca2+. Esto da como resultado una hipercontracción del sarcómero, la activación de la calpaína y la cPLA2, todo lo cual contribuye a aumentar el daño. Para más detalles, consulte el texto. Imagen creada con Bio Render y editada por Oscar A Ramírez Ruiz.
Edema
La inyección intramuscular de PLA2 del veneno de serpiente induce inflamación, caracterizada por un aumento de la permeabilidad vascular, formación de edema, reclutamiento de leucocitos a los tejidos afectados y liberación de mediadores inflamatorios [116]. Sin embargo, cabe señalar que los mecanismos por los cuales estas toxinas inducen este efecto no se comprenden completamente. Las PLA2 Asp49 pueden generar ácido araquidónico que sirve como punto de partida para producir eicosanoides que amplifican la respuesta inflamatoria. Por el contrario, los homólogos de PLA2 inducen edema y degranulación de mastocitos de una manera catalíticamente independiente. Es evidente que la actividad enzimática no es estrictamente necesaria para causar estos efectos. Una variedad de moléculas endógenas, incluyendo histamina, 5-hidroxitriptamina, bradicinina, taquicininas, metabolitos de ácido araquidónico, citocinas proinflamatorias y óxido nítrico (NO), han sido implicadas en la actividad inflamatoria de las PLA2 del veneno [116,117]. Otras características estructurales de las PLA2 también están implicadas en la inducción de la inflamación. Sin embargo, se necesitan más estudios para dilucidar estas regiones moleculares [116,117].
Neurotoxicidad
La neurotoxicidad presináptica o β-neurotoxicidad es otro efecto relevante observado en algunas mordeduras de serpientes. Por ejemplo, las infligidas por serpientes cascabel sudamericanas (Crotalus durissus) y las serpientes coral (especies de Micrurus; ver Capítulo 9) [113]. Las toxinas responsables de este efecto se denominan β-neurotoxinas, y sus estructuras poseen una, dos, tres y hasta cinco subunidades que pueden interactuar mediante enlaces covalentes o no covalentes. Una de sus subunidades es una PLA2 catalíticamente activa [118]. La toxicidad surge de la inhibición de la liberación de acetilcolina (Ach), lo que lleva a una parálisis flácida de los músculos respiratorios, incluido el diafragma. La inhibición de la liberación de Ach ocurre en tres pasos: primero, hay una ligera inhibición transitoria de la liberación de Ach que está relacionada con la unión de PLA2 a la membrana presináptica; segundo, hay un aumento significativo en la liberación de Ach; y finalmente, hay una inhibición sostenida de la liberación de Ach [112,119,120].
Los eventos celulares involucrados en la neurotoxicidad presináptica inducida por PLA2 del veneno de serpiente incluyen la unión de la toxina a una célula diana (motoneurona). Aunque la identificación precisa del receptor aún no se ha realizado, Lambeau et al. [121] encontraron una proteína de alta afinidad en el cerebro de rata, llamada tipo N debido a su origen neuronal. Estos receptores pueden estar ubicados cerca de la región donde se libera Ach. Después de la unión, PLA2 hidroliza los glicerofosfolípidos en la membrana presináptica, lo que lleva a una acumulación de ácidos grasos y lisofosfolípidos [119,122].
La capa externa de la membrana presináptica tiene una alta concentración de lisofosfolípidos que induce una curvatura y promueve la fusión de la membrana de la vesícula sináptica al tiempo que inhibe la endocitosis. Un experimento que agregó mezclas de ácidos grasos y lisofosfolípidos en las uniones neuromusculares respalda esta observación. Los efectos fueron similares a los inducidos por las PLA2 neurotóxicas [123]. La desestabilización de la membrana neuronal induce una afluencia de iones Ca2+ que pueden activar las PLA2 endógenas y las calpaínas. Esta activación puede causar la degradación interna de la membrana y la desorganización del citoesqueleto. Además, la alta concentración de calcio en el citosol puede desestabilizar las mitocondrias y alterar sus procesos bioquímicos [124]. Finalmente, la toxina es internalizada por las neuronas.
Sin embargo, el mecanismo por el cual las β-neurotoxinas son transportadas a las células nerviosas sigue siendo desconocido. Estas toxinas podrían ser internalizadas a través de poros generados después de la hidrólisis de la membrana presináptica, o las proteínas podrían usar la endocitosis de vesículas sinápticas como vehículo para entrar en las células nerviosas [125]. Una vez que la PLA2 entra en la neurona, se une a ciertas proteínas como la calmodulina, la proteína disulfuro isomerasa y proteínas de nombre 14-3-3; pero las implicaciones específicas para el modo de acción de la toxina no son claras. De manera similar, la calmodulina estabiliza la capacidad de la PLA2 para hidrolizar glicerofosfolípidos [126]. Además, las proteínas 14-3-3 desempeñan un papel importante en la dirección de las PLA2 a las vesículas sinápticas para llevar a cabo su actividad catalítica y posteriormente inhibir la endocitosis de vesículas [127]. En última instancia, estos eventos contribuyen a la falla de la unión neuromuscular, lo que resulta en la inhibición de la liberación de Ach y la posterior parálisis flácida [118–121,128,129] (Figura 10).
La crotoxina (CTX) es una β-neurotoxina derivada de Crotalus durissus, la serpiente cascabel sudamericana [138]. Consiste en una subunidad básica PLA2 conocida como CB y una subunidad ácida llamada crotapotina (subunidad A o CA). Esta última actúa como chaperona, guiando la subunidad CB a las células diana (motoneuronas) y evitando la unión de la PLA2 a sitios no específicos [131,132]. Además, CA aumenta la actividad neurotóxica de CB pero disminuye su actividad enzimática [133,134]. Por lo tanto, el complejo de crotoxina es responsable de la neurotoxicidad observada en el envenenamiento por la serpiente cascabel colombiana. Otras toxinas encontradas en venenos de especies de Colombia, clasificadas como β-neurotoxinas, incluyen las PLA2 aisladas de los venenos de las serpientes coral Micrurus mipartitus y M. dumerilii [135].

Figura 10. Representación esquemática de los eventos celulares que ocurren durante la neurotoxicidad inducida por PLA2 del veneno de serpiente. TEC: cadena de transporte de electrones. Las PLA2 pueden entrar en las vesículas presinápticas e hidrolizarlas (paso 1 y 2). La desestabilización de la membrana puede provocar una entrada masiva de calcio que puede activar enzimas dependientes del calcio, como las calpaínas, que promueven la hidrólisis del citoesqueleto (paso 3). PLA2 se internalizan a la terminal nerviosa para perpetuar el daño (paso 4). Para más detalles, consulte el texto. Imagen creada con Bio Render y editada por Oscar A Ramírez Ruiz.
Actividad anticoagulante
Las enzimas PLA2, según su potencia anticoagulante, se clasifican en enzimas fuertes, débiles y no anticoagulantes. Las PLA2 fuertemente anticoagulantes inhiben la coagulación sanguínea a concentraciones bajas (<2 µg/mL), mientras que las PLA2 débilmente anticoagulantes muestran efectos entre 3 y 10 µg/mL. Algunas PLA2 del veneno no prolongan significativamente los tiempos de coagulación, incluso a 15 µg/mL; y, por lo tanto, se clasifican como enzimas no anticoagulantes [138].
Los primeros estudios sobre PLA2 sugieren que la actividad catalítica es necesaria para sus efectos anticoagulantes [137]. Sin embargo, estudios recientes proponen que las PLA2 fuertemente anticoagulantes actúan a través de mecanismos tanto enzimáticos como no enzimáticos, siendo estos últimos mediados por un «sitio anticoagulante». Kini et al. [138] propusieron la posición de este sitio en la estructura tridimensional de las PLA2, que se encuentra entre los residuos 53 y 76, y está cargada positivamente, mientras que las PLA2 con actividad débil o no anticoagulante tienen un predominio de cargas negativas o neutras en esta región [136,138].
Sin embargo, Stefansson et al. [139] propusieron un mecanismo no enzimático para las PLA2 anticoagulantes [139]. El estudio demuestra que una PLA2 del veneno de la cobra escupidora de cuello negro (Naja nigricollis) que tiene fuertes propiedades anticoagulantes, se une al factor de coagulación Xa, bloqueando la activación adicional de la protrombina. Faure et al. [149] propusieron un modelo estructural para la interacción entre las PLA2 y el factor de coagulación Xa. Varios enfoques bioinformáticos proponen que los residuos específicos 2, 3 y 7 de la hélice 1; 16, 18, 19, 23, 24, 31–34 del bucle de unión a Ca2+; 53, 59, 60, 69, 70 de la hélice 3; y 118, 119, 121–124, 129–131 y 133 de las láminas β, se unen entre sí. El conocimiento aceptado actualmente propone que las PLA2 del veneno de serpiente pueden provocar su actividad anticoagulante a través de mecanismos tanto enzimáticos como no enzimáticos [141,142].
Otras actividades biológicas inducidas por efecto de PLA 2
Las PLA2 del veneno pueden modular la agregación plaquetaria, ya sea induciéndola o inhibiéndola. Se clasifican en tres clases distintas: A, B y C [143]. Las enzimas de clase A inician la agregación plaquetaria [144,145]; las PLA2 de clase B causan inhibición de la agregación plaquetaria [146,147]; y las PLA2 de clase C muestran efectos bifásicos que inducen la agregación plaquetaria en dosis bajas o tiempos de incubación cortos, mientras que inhiben la agregación plaquetaria en concentraciones más altas o incubación prolongada [148].
Además, estas toxinas tienen efectos hipotensores, sin embargo, el mecanismo molecular y celular responsable de la reducción de la presión arterial no se comprende completamente [149]. Por ejemplo, la BthA-I-PLA2, aislada de la víbora de foseta jararacussu (Bothrops jararacussu), reduce la presión arterial debido a su actividad de fosfolipasa. Esto fue apoyado por la alquilación de His48 con bromuro de p-bromofenacilo que bloquea el mecanismo catalítico de la enzima [150]. Por otro lado, las toxinas PLA2 OSC3a y OSC3b aisladas de la taipán de Papúa (Oxyuranus scutellatus), producen efectos hipotensores al generar metabolitos de ciclooxigenasa (prostaglandinas dilatadoras o prostaciclina) que pueden estar involucrados en la liberación de mediadores endógenos, como la histamina y la bradiquinina [151]. Sin embargo, no se ha informado el modo de acción de otras enzimas, como BmooPLA2-I, de la cuatronarices brasileña (Bothrops moojeni) [152].
Las PLA2 del veneno de serpiente exhiben actividades citotóxicas, bactericidas y antivirales. Algunas toxinas provocan estas actividades de manera dependiente del catalizador. Sin embargo, también se ha reportado que los homólogos de PLA2 tienen estos efectos, lo que sugiere que la actividad catalítica no está completamente involucrada en la inducción de dichos efectos biológicos [153,154]. Se necesitan más estudios para revelar los mecanismos moleculares involucrados en la inducción de estos efectos.
2.4. L-Aminoácido Oxidasas (LAAOs) del veneno de serpiente
Las L-aminoácido oxidasas están ampliamente presentes en los venenos de serpientes, así como en otros organismos [155–157]. Estas flavoproteínas son responsables del color amarillo en los venenos de serpientes. Catalizan la desaminación oxidativa estereoespecífica de los L-aminoácidos, produciendo el alfa-cetoácido, amoníaco y peróxido de hidrógeno (H202) (Figura 11). Las LAAO son proteínas homodiméricas que constan de tres dominios (Tabla 1). Cada monómero tiene una masa molecular de 57-68 kDa. La enzima normalmente constituye entre el 1% y el 4% del peso del veneno. Sin embargo, en ciertas especies, como la víbora malaya (Callosellasma rhodostoma), la enzima puede representar hasta el 30% del peso seco del veneno [167]. La presencia de LAAO en los venenos de serpientes y sus actividades biológicas pueden mejorar las acciones de otras toxinas importantes y contribuir a la inmovilización de la presa o al aumento de la toxicidad en las mordeduras de serpiente.

Figura 11. Reacción catalizada por L-aminoácido oxidasas (LAAO) del veneno de serpiente.
Tabla 1. Dominios presentes en L-aminoácido oxidasas (LAAO) del veneno de serpiente
|
Dominios
|
Rango de residuos de aminoácidos
|
|
Dominio de unión a FAD
|
35-64, 241-318, 446-471
|
|
Dominio de unión a sustrato
|
5-25,73-129, 233-235,323-420
|
|
Dominio helicoidal
|
130-230
|
El dominio de unión al dinucleótido de flavina y adenina (FAD) estabiliza la molécula FAD+ necesaria para la catálisis. La estructura secundaria de este dominio consta de seis láminas β y cinco hélices α con la adición de dos láminas β cortas y una hélice α. Cuatro de las seis láminas β son paralelas y las dos restantes son antiparalelas. Ambas láminas β cortas son paralelas entre sí. Este dominio contiene la secuencia de consenso de residuos de glicina (G40XG42XXG45) que participa en el acceso al grupo fosfato cargado negativamente de la coenzima. Además, varios puentes salinos estabilizan este dominio [159,160]. El dominio de unión al sustrato interactúa con el aminoácido que se va a oxidar y contiene seis hélices α y once láminas β[159,160]. Finalmente, el dominio helicoidal es el único con una secuencia continua. La estructura secundaria del dominio consta de seis hélices α con una hélice α corta y varios bucles [159,160] (Figura 12).

Figura 12. Estructura de L-aminoácido oxidasa (LAAO) del veneno de serpiente. (A) El dominio de unión a FAD se muestra en azul. El dominio de unión al sustrato se muestra en cian (azul verdoso) y el dominio helicoidal se presenta en magenta. Las glicosilaciones se representan como barras. (B) Un residuo de fenilalanina se muestra en bolas y barras en el sitio activo, mientras que el FAD se representa como un alambre. Figura preparada a partir del código PDB 2IID.
El residuo más importante para la catálisis es la His223, que se conserva en todas las LAAO encontradas en venenos de serpiente [166,168]. Este residuo actúa como nucleófilo al desprotonar el α-amino del sustrato (aminoácido) [157,159]. Además, este aminoácido tiene dos conformaciones, A y B. Durante la catálisis, la His223 pasa el 40% de su tiempo en la conformación A y el 60% en la B. Como conformación A, este residuo impide la entrada de oxígeno; mientras que, en la conformación B, el oxígeno puede entrar libremente y participar en la formación final de peróxido de hidrógeno para recuperar el FAD+[161]. Aunque las LAAO oxidan todos los L-aminoácidos, prefieren los aminoácidos aromáticos e hidrofóbicos, como la fenilalanina y la leucina [159,160,162].
Es importante destacar que estas toxinas son glicoproteínas. En algunos casos, se sabe que la eliminación de carbohidratos reduce los efectos enzimáticos y biológicos causados por las LAAO del veneno de serpiente [163,164]. Sin embargo, la desglicosilación no afecta las actividades enzimáticas de las LAAO de las víboras de foseta como Bothrops pauloensis, B. jararaca, B. alternatus y B. moojeni [165–167]. Por lo tanto, se necesitan más estudios para determinar el papel preciso de la glicosilación en las LAAO del veneno de serpiente y sus implicaciones en los efectos biológicos inducidos. Se conocen algunas LAAO en el veneno de especies colombianas de importancia médica, entre ellas Crotalus durissus, Bothriechis schlegelii y Micrurus mipartitus [135,168,169]. Estas enzimas inducen varios efectos biológicos, que se describen a continuación [135,168–170].
Actividades biológicas inducidas por LAAO del veneno de serpiente
Los efectos biológicos de las LAAO se atribuyen al peróxido de hidrógeno (H202) generado en la reacción catalítica. Los efectos son inhibidos por los depuradores de H202 como la catalasa [171–174]. Sin embargo, varios estudios demuestran que los efectos biológicos no se recuperan completamente mediante la incubación con catalasa, lo que sugiere que los efectos producidos por las LAAO no se deben únicamente a la producción de H202 [157,175,176].
Hemorragia
Algunas LAAO que se encuentran en los venenos de serpiente pueden inducir hemorragia por sí mismas [165,177–179]. Este efecto se atribuye a la capacidad de las LAAO para inducir apoptosis en células endoteliales. Esto conduce a la ruptura del endotelio y la extravasación de glóbulos rojos. La acumulación de H202 en los vasos sanguíneos puede ser responsable del efecto apoptótico sobre las células endoteliales [177]. Por lo tanto, se sugiere que este efecto contribuye a la actividad hemorrágica provocada por las SVMP.
Edema
El mecanismo preciso que produce este efecto, por parte de las LAAO, no se entiende por completo. Una LAAO de la serpiente japonesa mamushi (Gloydius blomhoffii) estimula los linfocitos y monocitos para liberar citocinas proinflamatorias, IL-6, IL-2 e IL-12, lo que puede explicar la actividad inflamatoria de esta enzima [180]. Sin embargo, Izidoro et al. [171] sugirieron que la formación de edema se debe a la activación de la respuesta inflamatoria por el H202 generado, ya que la administración de glutatión (un antioxidante) a la pata del ratón inhibe la actividad inductora de edema de la enzima [181].
Modulación de la agregación plaquetaria
El efecto de las LAAO de veneno de serpiente sobre la agregación plaquetaria no es concluyente. Algunas enzimas inducen agregación plaquetaria, mientras que otras toxinas producen inhibición de la agregación de estas células [162]. Du y Clemetson [182] propusieron que el peróxido de hidrógeno generado en la reacción catalítica de las LAAO es responsable de inducir e inhibir la agregación plaquetaria. La actividad inhibidora puede estar relacionada con el peróxido de hidrógeno que impide la interacción entre el fibrinógeno y su receptor en la superficie de la plaqueta (GPIIb/IIIa) o reduce la unión del ADP a las plaquetas [183,184]. Por otro lado, la activación plaquetaria puede ser una consecuencia de la producción de tromboxano A2 provocada por H202 [172]. A pesar de esta evidencia, se requieren más estudios sistemáticos para determinar por qué las LAAO pueden provocar o inhibir la agregación plaquetaria.
Otras actividades biológicas que no están relacionadas con el envenenamiento por mordedura de serpiente
Las LAAO inducen varias actividades que las hacen atractivas para la búsqueda de alternativas terapéuticas en infecciones protozoarias, virales, bacterianas y en cáncer, debido a su citotoxicidad (ver Capítulo 10). Varias LAAO de veneno de serpiente tienen actividad antileishmaniasis [171–173,185–187]. Este efecto se atribuye al H2O2 generado por el ciclo catalítico de las enzimas.
Asimismo, una LAAO de veneno de serpiente aislada de Trimeresurus stejnegeri (víbora verde china) tiene actividad antiviral contra el VIH-1 [179]. Las LAAO también han demostrado actividad antibacteriana contra bacterias gramnegativas y grampositivas [162,168,181,188]. El modo de acción de las LAAO para inducir el efecto antimicrobiano se le atribuye al H2O2. Sin embargo, la unión de la enzima a la superficie bacteriana y una mayor concentración de H2O2 parecen ser esenciales para este efecto [189]. Varias LAAO derivadas del veneno de serpientes muestran actividad citotóxica contra líneas de células tumorales [199], además, pueden inducir apoptosis en estas células. Este efecto involucra al H2O2, pero también se especula que las fracciones de glicano son cruciales para la interacción con la célula objetivo [191].
2.5. Desintegrinas del veneno de serpientes
El término «desintegrina» fue introducido en 1987 por primera vez en la toxinología por Huang et al. [192], al describir, aislar y caracterizar la trigamina del veneno de Craspedocephalus gramineus (víbora común del bambú). Sin embargo, hoy en día este término se utiliza para definir toxinas con una longitud de entre 40 y 100 aminoácidos presentes en el veneno de vipéridos. Estas toxinas se producen por procesamiento proteolítico de las SVMP P-II y P-III. Inicialmente, el motivo Arg-Gly-Asp (RGD) se describió como una característica estructural esencial para su actividad principal, que es inhibir la agregación plaquetaria mediante el bloqueo de las integrinas β1 y β3 [198].
Estructura de las desintegrinas del veneno de serpiente
Las desintegrinas del veneno de serpiente se pueden clasificar en cuatro grupos según su número de aminoácidos y enlaces disulfuro [193,194]. El primer grupo está formado por desintegrinas cortas de 41 a 51 residuos y cuatro enlaces disulfuro. El segundo grupo está formado por desintegrinas de tamaño medio que contienen alrededor de 70 aminoácidos y seis enlaces disulfuro. El tercer grupo está formado por péptidos largos con aproximadamente 84 aminoácidos y siete enlaces de cisteína que los entrecruzan. El cuarto grupo incorpora homo y heterodímeros. Estas desintegrinas están compuestas por 67 aminoácidos y diez residuos de cisteína, que intervienen en la estabilización de la estructura mediante la formación de enlaces disulfuro intra e intercatenarios.
El motivo RDG es el más común en las desintegrinas de veneno de serpiente. Sin embargo, también se han identificado otros motivos. La Tabla 2 describe la diversidad de estos motivos y sus dianas moleculares (principalmente, integrinas). Los estudios estructurales de desintegrinas de tamaño corto, mediano, grande y diméricas revelaron un bucle móvil que contiene el motivo activo, que sobresale 14–17 Å del núcleo de la proteína [193,194,195] (Figura 13).
Tabla 2. Integrinas, su función y desintegrinas del veneno de serpiente que las inhiben.
|
Integrina
|
Función
|
Desintegrinas que la inhiben*
|
Referencias
|
|
α5β1
|
Principal receptor de fibronectina
|
RGD, VGD y MDG
|
[145,147,148,193,197,198]
|
|
α8β1
|
Receptor de tenascina
|
RGD
|
|
αvβ1
|
Receptor de vitronectina
|
RGD
|
|
αvβ3
|
Receptor de vitronectina
|
RGD, WGD
|
|
αIIbβ3
|
Receptor de fibrinógeno plaquetario implicado en la agregación plaquetaria
|
RGD, WGD y KGD
|
|
α4β1
|
Fibronectina y receptor VCAM-1
|
MLD
|
|
α4β7
|
Fibronectina, receptor VCAM-1 y receptor MdCAM
|
MLD
|
|
α3β1
|
Receptor de laminina
|
MLD
|
|
α6β1
|
Receptor de laminina
|
MLD
|
|
α7β1
|
Receptor de laminina
|
MLD
|
|
α9β1
|
Receptor de tenascina
|
MLD
|
|
α1β1
|
Receptor de colágeno IV
|
KTS y RTS
|

Figura 13. Estructura de las desintegrinas del veneno de serpiente. (A) Desintegrina monomérica. (B) Desintegrina heterodimérica. Las cadenas azul y roja representan diferentes subunidades. El motivo RGD se muestra en esquema de varitas. Las letras N y C representan el extremo N y C de cada cadena, respectivamente. Esta imagen se preparó a partir de las estructuras PDB con los códigos 2MOP (monomérica) y 1TEJ (heterodimérica).
Actividades biológicas inducidas por las desintegrinas del veneno de serpiente
Debido a su capacidad para bloquear las integrinas, la actividad más importante inducida por las desintegrinas del veneno de serpiente es la inhibición de la agregación plaquetaria. Esto puede contribuir a los trastornos hemostáticos causados por las SVSP, SVMP y otras toxinas en el envenenamiento por mordedura de serpiente [199–203]. De hecho, la actividad antiagregación plaquetaria de las desintegrinas las ha convertido en plantillas esenciales para el desarrollo de fármacos utilizados como agentes antitrombóticos. Por ejemplo, el tirofibán se deriva de la echistatina que se encuentra en el veneno de Echis carinatus (serpiente Phoorsa) [204] y el eptifibatide se obtiene de la barbourina presente en el veneno de Sistrurus miliarius barbouri [200,201,205] (ver Capítulo 10).
Además, las desintegrinas del veneno de serpiente han demostrado potencial en la terapia contra el cáncer. Pueden actuar sobre una amplia variedad de integrinas y, por lo tanto, es plausible que tengan el potencial de interferir en procesos esenciales involucrados en la carcinogénesis, el crecimiento tumoral, la invasión y migración celular (ver Capítulo 10). Por lo tanto, las desintegrinas pueden utilizarse como herramientas y posibles fármacos para el tratamiento de este problema de salud [197,206–208].
2.6. Toxinas de tres dedos (3FTx)
Estructura
La familia de proteínas conocida como toxinas de tres dedos está formada por polipéptidos no enzimáticos que van desde 60 a 62 residuos de aminoácidos (cadena corta) y entre 66 y 74 residuos de aminoácidos (cadena larga). Por lo general, son monómeros y contienen de 4 a 5 puentes disulfuro altamente conservados. Su plegamiento se caracteriza por tres bucles con cadenas beta que parten de un núcleo hidrofóbico que a su vez está atravesado por cuatro enlaces disulfuro. Los venenos de elápidos, hidrófidos (serpientes marinas) y colúbridos contienen estas proteínas [209].
La estructura primaria de una proteína está determinada por su secuencia de aminoácidos que se representan como cuentas en un collar. Para tener una estructura completa y funcional, son necesarias interacciones específicas entre aminoácidos para un plegamiento adecuado. La Figura 14 muestra la estructura primaria y terciaria de la mipartoxina-I, una toxina que se encuentra en el veneno de Micrurus mipartitus (rabo de ají).

Figura 14. La estructura primaria de la mipartoxina-I del veneno de Micrurus mipartitus (código GenBank: AVI57319.1) se representa como una cadena larga. Las interacciones entre los residuos de aminoácidos facilitan el plegamiento en varios sitios de la cadena, lo que contribuye a la conformación de la estructura secundaria y terciaria. La mipartoxina-I tiene una estructura tridimensional que se asemeja a los tres dedos centrales de una mano. Es por eso que pertenece a la familia de proteínas conocidas como toxinas de tres dedos (3FTx) [210].
Aunque los miembros de la familia 3FTx comparten características estructurales conservadas que les permiten plegarse y mantener su integridad, también ocurren algunas modificaciones. Estas modificaciones pueden estar relacionadas con la diversidad de sus funciones y su afinidad por ciertas dianas moleculares [210,211]. Además de los ocho residuos de cisteína presentes en la región central que favorecen la formación de puentes disulfuro, los residuos aromáticos de tirosina o fenilalanina en las posiciones 25 y 27 también se conservan y contribuyen al adecuado plegamiento y estabilidad de la estructura de la lámina beta en esta toxina. La estabilidad de la conformación nativa también es aportada por los residuos de arginina en la posición 39 y ácido aspártico en la posición 60, ya que forman puentes salinos con cualquiera de los extremos terminales [209,212]. La Tabla 3 enumera las principales variaciones reportadas descritas para las estructuras 3FTx.
Tabla 3. Algunos ejemplos de las variaciones estructurales encontradas en la familia de toxinas de tres dedos.
|
Variación / Estructura tridimensional
|
Nombre de la toxina
|
Especie de serpiente
|
Anotaciones
|
Referencias
|
|
Cadena corta
|
Erabutoxina
(código PDB 1QKE)
|
Laticauda semifasciata
Serpiente marina china (Elapidae)
|
62 aminoácidos
|
[213]
|
|
Cadena larga
|
Bungarotoxina (código PDB 1IKC)
|
Bungarus multicinctus
Krait con muchas bandas de Taiwán (Elapidae)
|
74 aminoácidos
|
[214]
|
|
Presencia de un quinto enlace disulfuro en el bucle I
|
WTX recombinante
(código PDB 2MJ0)
|
Naja kaouthia
Cobra de monóculo del sur y sudeste de Asia (Elapidae)
|
Aunque anteriormente se la clasificaba como una neurotoxina débil, estudios recientes muestran que las toxinas con un quinto enlace disulfuro en esa posición son altamente tóxicas y se unen a los receptores nicotínicos de acetilcolina en concentraciones en el rango nanomolar. Estas toxinas se denominan «3FTx no convencionales» debido a su diferente selectividad y especificidad con respecto a las neurotoxinas canónicas. Un ejemplo adicional es la toxina BM14 que se encuentra en el veneno de
Bungarus multicintus de Taiwán. Esta toxina exhibe una mayor afinidad por el receptor muscarínico de acetilcolina M2 que por el M1
|
[215,216]
|
|
Presencia de un quinto enlace disulfuro en el bucle II
|
NTX-1
(código PDB 1NTN)
|
Naja oxiana
Cobra de Asia Central (Elapidae)
|
El quinto enlace disulfuro en el bucle II proporciona un giro y forma un segmento helicoidal corto en la punta del bucle. Esta unión adicional desempeña un papel importante en la especificidad del bloqueo del receptor nicotínico de acetilcolina α7. Sin embargo, toxinas como la candoxina y la haditoxina que carecen de esta variación también se unen a este receptor
|
[217]
|
|
Extensiones en el N-terminal o C-terminal
|
Denmotoxina
(código PDB 2H5F)
|
Boiga dendrophila
Serpiente de manglar o serpiente gato de anillos dorados de Malasia (Colubridae)
|
La proteína tiene un segmento N-terminal extendido con siete residuos adicionales, que están cubiertos por un ácido piroglutámico.
Actualmente se desconoce el efecto de esta variación estructural sobre su función. Normalmente, las neurotoxinas de cadena larga tienen entre 2 y 9 residuos adicionales en el extremo C.
|
[218]
|
|
Dímeros con enlaces no covalentes
|
Haditoxina
(código PDB 3HH7)
|
Ophiophagus hannah
La cobra real desde la India hasta el sudeste asiático (Elapidae)
|
La mayoría de las 3FTx son monómeros, pero algunos pueden existir de forma natural como dímeros. La haditoxina es un homodímero, con dos monómeros de cadena corta que están unidos de forma no covalente y orientados en direcciones opuestas. Esto le da a la haditoxina la capacidad de funcionar como antagonista de los receptores nicotínicos de acetilcolina musculares (αβγδ) y neuronales (α7, α3β2 y α4β2)
|
[219]
|
|
Dímeros unidos de manera covalente
|
Irditoxina (código PDB 2H7Z)
|
Boiga irregularis
Serpiente arbórea parda de Australia, Papúa Nueva Guinea y las islas del noroeste de Melanesia (Colubridae)
|
El heterodímero consta de dos subunidades 3FTx no convencionales, cada una de las cuales contiene una cisteína adicional que forma un enlace disulfuro entre cadenas. Además, el extremo N-terminal tiene una extensión de siete residuos de aminoácidos. Esto da como resultado una potente neurotoxina postsináptica.
Otros ejemplos incluyen el heterodímero α-cobrotoxina/citotoxina, que se une al receptor nicotínico de acetilcolina muscular α7 pero no tiene actividad citotóxica, y el homodímero de α-cobrotoxina que reconoce el receptor nicotínico de acetilcolina neuronal α3β2 como la β-bungarotoxina.
|
[218]
|
|
Toxinas sinérgicas
|
SynTx
(código PDB 7C28)
|
Dendroaspis jamesoni
Mamba verde del Congo (Elapidae)
|
La molécula es un homodímero conectado por un enlace disulfuro intercatenario. La dimerización ocurre a través de los bucles II y III de los monómeros.
Aunque este grupo contiene moléculas con una secuencia de aminoácidos similar a otras neurotoxinas y citotoxinas de la familia 3FTx, su toxicidad puede ser baja o nula. Sin embargo, su importancia radica en que incrementa la toxicidad de otras proteínas del veneno (sinergismo), como las toxinas tipo angusticeps, que, a su vez, se comportan como inhibidores de la colinesterasa.
|
[5,221,222]
|
Modelos tridimensionales tomados de PDB y preparados en UCSF Chimera v.1.17.3. Todas estas toxinas tienen una vista de modelo 3D disponible en https://serpientes.ins.gov.co/
Recientemente, algunos autores propusieron una guía para separar y estudiar sistemáticamente las toxinas de tres dedos debido a la alta variabilidad entre las secuencias de aminoácidos de estas proteínas [223–225]. Reunieron una base de datos con secuencias 3FTx seleccionadas conocidas para especies de Micrurus de Brasil y clasificaron las proteínas en función de su similitud e identidad estructural. En consecuencia, los autores establecieron parámetros para proponer una reclasificación basada en aminoácidos funcionales clave, puentes disulfuro, distribución de carga en la superficie y la relación filogenética de la secuencia [223–225]. La funcionalidad de la proteína se puede atribuir a nueve grupos con base en la homología de sus secuencias. Esta clasificación sirve como referencia para las toxinas de tres dedos encontradas en especies de Micrurus de Suramérica únicamente.
Los modelos predichos a partir de las secuencias primarias de especímenes brasileños se basaron en secuencias homólogas de elápidos del viejo mundo, incluidos Dendroaspis polylepis, Naja atra, Bungarus multicinctus, B. candidus, Ophiophagus hannah, Dendroaspis jamesoni y Hemachatus haemachatus (cobra escupidora de cuello anillado). Solo se construyó una secuencia a partir de la fulditoxina del veneno de Micrurus fulvius (código PDB 4RUD). Los nueve grupos recientemente conformados incluyeron especímenes locales representativos como Micrurus altirostris, Micrurus carvalhoi, Micrurus corallinus, Micrurus frontalis, Micrurus lemniscatus, Micrurus paraensis, Micrurus spixii y Micrurus surinamensis [224].
Solo se ha purificado y caracterizado una pequeña cantidad de toxinas de tres dedos a partir de venenos de Micrurus colombianas. Actualmente, solo tres toxinas tienen modelos tridimensionales predichos a partir de secuencias reportadas en la literatura. Dos de estas toxinas pertenecen a especies de serpientes de importancia médica en Colombia (M. mipartitus y M. dumerilii; Tabla 4) [225]. Además, ejemplifican los grupos de patrones bicolor y de mónadas, respectivamente [235]. Otra especie del grupo de patrones de mónadas, M. clarki, también ha sido objeto de investigación de 3FTx [227].
Tabla 4. Estructuras de toxinas de tres dedos purificadas de algunos venenos de Micrurus en Colombia. A cada secuencia se le asigna un número de acceso en Uniprot
|
Nombre de las toxinas de tres dedos y número de acceso en UniProt
|
Especie de serpiente
|
Secuencia
|
Modelo 3D predicho
|
Referencias
|
|
Clarkitoxina-I-Mdum
C0HLK5
|
Micrurus dumerilii
Coral capuchina
Elapidae
|
RICDDSSIPFLRTPQLCPKGQDVCYKKTPIVKKFKWLQKKGCASSCPKDGFIKIFKIECCTKDNCI
|

|
[226]
|
|
Mipartoxina-1
B3EWF8
|
Micrurus mipartitus
Coral rabo de ají
|
MKTLLLTLVVVTIVCLDLGNSLKCYVSREGKTQTCPEGEKLCEKYAVSYFHDGRWRYRYECTSACHRGPYNVCCSTDLCNK
|

|
[226]
|
Clarkitoxina-1
C0HK04
|
Micrurus clarki
|
RICDDSSIPFLRTPQLCPKGQDVCYKKTPIVKKFKWLQKKGCASSCPKNGFIKIFKIECCTKDNCI
|

|
[227]
|
Secuencias utilizadas para predecir las estructuras tridimensionales presentadas mediante el software SWISS MODEL [239,240]. Moléculas ajustadas y preparadas en UCSF Chimera v.1.17.3
Actividades biológicas
Aunque las toxinas de la familia 3FTx comparten una similitud estructural, exhiben una amplia variedad de actividades fisiopatológicas y mecanismos de acción, incluyendo neurotoxicidad postsináptica, citotoxicidad, cardiotoxicidad, efecto anticoagulante y antiplaquetario. Además, actúan como antagonistas del canal de Ca2+ tipo L y pueden promover sinérgicamente la hipotensión con las toxinas muscarínicas [223]. Las 3FTx tienen una estructura pequeña, compacta y estable con múltiples sitios de interacción para lograr varias funciones que han sido optimizadas evolutivamente [223].
Neurotoxinas
La neurotoxicidad es posiblemente el efecto más conocido de las 3FTx debido al drástico efecto de parálisis flácida e insuficiencia respiratoria en víctimas de mordeduras de serpiente. La interferencia con la transmisión de la señal puede ocurrir por diferentes vías. En el caso de la transmisión colinérgica alterada en los sistemas nerviosos central y periférico, la neurotoxicidad puede ocurrir en varios sitios en la región postsináptica, afectando tanto a los receptores nicotínicos como a los muscarínicos [15] (Figura 15). Los siguientes son ejemplos de toxinas y sus mecanismos de acción que conducen a neurotoxicidad:
Toxinas similares al curare o α-neurotoxinas.— Son similares al alcaloide curare (del extracto de plantas de pueblos indígenas de América Central y del Sur). Son neurotoxinas de cadena larga y corta que se unen al receptor de acetilcolina muscular α1 e impiden la transmisión neuromuscular efectiva. En contraste, las neurotoxinas de cadena larga se unen con alta afinidad al receptor de acetilcolina neuronal α7.
Toxinas muscarínicas.— Estas comprenden toxinas que actúan como agonistas o antagonistas de los receptores muscarínicos. La toxina muscarínica α (MTα) aislada del veneno de mamba (Dendroaspis jamesoni) es un potente antagonista del adrenoceptor α2B [212,223].
α-neurotoxinas.— Estas se unen específicamente al receptor de acetilcolina nicotínico neuronal (α3β4). Su estructura se asemeja a la de las α-neurotoxinas de cadena larga, pero se presentan como dímeros. No reconocen el receptor de acetilcolina α1.
Recientemente, las micrurotoxinas 1 y 2 (MmTX1 y MmTX2), aisladas del veneno de Micrurus mipartitus (rabo de ají), se han descrito como agonistas del receptor ionotrópico GABAA que mejora la apertura y la desensibilización del receptor.

Figura 15. Las toxinas de tres dedos bloquean los receptores de acetilcolina en la célula efectora de la unión neuromuscular, lo que provoca parálisis flácida en los miocitos e insuficiencia respiratoria cuando se ve afectado el diafragma. Imagen creada con BioRender y editada por Oscar A Ramírez Ruiz.
Los inhibidores de la acetilcolinesterasa interfieren con la transmisión neuromuscular al inhibir la unión al sitio periférico de la enzima acetilcolinesterasa (AChE) presente en la unión neuromuscular. Esto evita que la AChE capture y degrade la acetilcolina, lo que lleva a espasmos musculares debido a la acumulación de acetilcolina en la hendidura sináptica. Estos inhibidores se conocen como fasciculinas y tienen una estructura similar a las neurotoxinas de cadena corta [209,212].
Cardiotoxinas
Las cardiotoxinas son un grupo de toxinas que se han encontrado exclusivamente en venenos de cobra, particularmente en especies del género Naja. Estas toxinas son similares a las neurotoxinas de cadena corta, contienen cuatro enlaces disulfuro y representan el segundo grupo más grande después de las neurotoxinas. Son responsables de aumentar la frecuencia cardíaca y pueden provocar un paro cardíaco de manera dependiente de la dosis. Además, muchas toxinas de este grupo también tienen efectos citolíticos, formando poros en las membranas lipídicas, por lo que también se les conoce como citolisinas. Finalmente, se reconoce otro subgrupo dentro de esta clasificación, las β-cardiotoxinas y toxinas relacionadas. Este subgrupo exhibe actividad bloqueadora de los receptores adrenérgicos cardíacos β1 y β2 [212].
Bloqueadores de los canales de calcio de tipo L
Estructuralmente, estos bloqueadores de canales son polipéptidos similares a las neurotoxinas de cadena corta. Se unen al sitio de unión de la 1,4-dihidropiridina de los canales de calcio de tipo L y bloquean físicamente las corrientes de calcio, lo que lleva a la relajación del músculo liso y la inhibición de las contracciones cardíacas [212].
Trastornos de la hemostasia
Solo unas pocas 3FTx tienen la secuencia de aminoácidos RGD (Arginina-Glicina-Ácido Aspártico) en un sitio accesible para la interacción. El tripéptido RGD participa en la función adhesiva de varias proteínas, interfiriendo en la interacción entre el fibrinógeno y su complejo receptor para la glicoproteína IIB-IIIa (αIIββ3) inhibiendo la agregación plaquetaria [223].
La toxina KT-6.9, aislada del veneno de la cobra Naja kaouthia, inhibe la agregación plaquetaria inducida por el difosfato de adenosina (ADP), la trombina y el ácido araquidónico. Como resultado, inhibe la agregación plaquetaria (hemostasia primaria) de manera dosis-dependiente [223]. En la hemostasia secundaria, el veneno de cobra de la especie Hemachatus haemachatus (Cobra escupidora de cuello anillado) contiene ringhalexina y exactina que inhiben la activación del factor de coagulación X, causando alteraciones de la coagulación [223].
Interacción con canales de sodio
Algunas 3FTx interactúan con los canales iónicos sensibles al ácido (ASIC), que normalmente son canales iónicos selectivos para Na+ y se activan con protones. Los ASIC están ampliamente distribuidos en todo el sistema nervioso periférico y central de los vertebrados. Estos canales están involucrados en la plasticidad sináptica, la neurodegeneración y la percepción del dolor [223].
2.7. Serinoproteasas del veneno de serpiente (SVSP)
Estructura
Las serinoproteasas son proteínas glicosiladas con actividad enzimática. Su peso molecular oscila entre 26 y 67 kDa, dependiendo del grado de N- u O-glicosilación. El subgrupo conocido como serinoproteasas tipo trombina es responsable de degradar el fibrinógeno y amplificar las condiciones de sangrado durante el envenenamiento [244]. En el veneno de Bothrops asper (cuatronarices, barbiamarilla) las serinoproteasas son constituyentes abundantes que representan entre el 5% y el 18% de las proteínas, dependiendo de la edad de la serpiente y su región geográfica [235]. Estas proteasas, junto con las hialuronidasas, metaloproteasas y fosfolipasas A2 pertenecen al grupo de enzimas hemotóxicas que son importantes en los venenos de víbora [232].
Estas proteasas se caracterizan por un pliegue típico de quimotripsina y dos barriles de láminas β de 6 hebras (Figura 16). Su sitio activo se encuentra en la hendidura entre estas últimas e incluyen una tríada catalítica canónica de His-Asp-Ser. Aunque comparten entre el 50% y el 80% de identidad en sus secuencias de aminoácidos, difieren significativamente en su especificidad de sustrato y, por lo tanto, en su actividad [233]. Varias SVSP tienen actividad farmacológica (ver Capítulo 10). Por ejemplo, las mambalginas son toxinas que se encuentran en el veneno de la mamba negra (Dendroaspis polylepis). Tienen efecto analgésico al bloquear los canales con las subunidades ASIC1a y ASIC2a en el sistema nervioso central, así como los canales que incluyen la subunidad ASIC1b en los nociceptores [229].

Figura 16. AaV-SP-I, serinoproteasa glicosilada presente en el veneno de la serpiente Agkistrodon acutus. Modelo tridimensional tomado de PDB (código 1OP0) y preparado en UCSF Chimera v.1.17.3
Actividades biológicas
Hemotóxico y coagulopático
El cuerpo humano puede contrarrestar el efecto hemorrágico producido por las metaloproteasas. Sin embargo, las serinoproteasas similares a la trombina, presentes en el veneno de los vipéridos, impiden la formación de un tapón de fibrina eficaz. En condiciones fisiológicas, la trombina endógena escinde regiones específicas de fibrinógeno en la circulación antes del sangrado. Esto expone el sitio de unión de otras moléculas de fibrinógeno que experimentan la misma hidrólisis, formando una malla de fibrina que estabiliza el coágulo y promueve la hemostasia.
Las serinoproteasas similares a trombina simulan la actividad de la trombina, pero hidrolizan diferentes sitios en la molécula de fibrinógeno. Esto altera la unión entre las moléculas de fibrinógeno, lo que da como resultado un consumo irregular de fibrinógeno y la formación de un coágulo inestable de fibrina (Figura 17) [234–237]. Si bien muchas serinoproteasas actúan sobre la cadena α del fibrinógeno, algunas purificadas de especies de Agkistrodon actúan tanto sobre la cadena α como sobre la β[238]. Además, este subgrupo de enzimas tiene la capacidad de activar los factores de coagulación VIII y XIII, así como la trombina. También son responsables de la activación de la proteína C, la agregación plaquetaria, la activación del plasminógeno y el factor V. Estas acciones pueden dar lugar a una coagulopatía de consumo (Figura 18) [238,239].

Figura 17. Modelo de alteración de la hemostasia. Las moléculas de fibrinógeno circulantes (representadas en azul) sufren una hidrólisis inadecuada por parte de las serinoproteasas similares a trombina, lo que favorece su consumo errático y excesivo, dando como resultado la formación de un coágulo deficiente de fibrina y una hemostasia insatisfactoria. Además, las plaquetas (estructuras en blanco) se agregan de manera inapropiada en sitios distintos al sitio de la lesión, lo que conduce a un consumo inadecuado, así como a un sangrado incontenible y la aparición de microtrombos. La imagen fue creada con Bio Render y editada por Oscar A Ramírez Ruiz.
La asperasa fue la primera serinoproteasa aislada del veneno de Bothrops asper. Es una enzima glicosilada con una masa molecular de 30 kDa. Esta proteína promueve la desfibrinogenación en modelos murinos y causa alteraciones del comportamiento, como pérdida del reflejo de enderezamiento, opistótonos (espasmo de los músculos que causa arqueamiento hacia atrás de la cabeza, el cuello y la columna vertebral) y rotaciones intermitentes a lo largo del eje longitudinal del cuerpo. Esta característica es similar a la de la giroxina, una enzima similar a la trombina que se encuentra en el veneno de Crotalus durissus de poblaciones brasileñas [236,240].

Figura 18. Esquema de las vías de la cascada de coagulación sanguínea en condiciones fisiológicas que indican los sitios respectivos de acción de las serinoproteasas del veneno de serpiente (SVSP). Las flechas rojas indican la activación por SVSP. La flecha negra indica la actividad de la fibrinogenasa, que causa un consumo inadecuado de fibrinógeno, y la flecha punteada resalta la actividad similar a la trombina (ver más información dentro del texto). Las SVSP actúan como enzimas de activación plaquetaria, lo que permite una agregación excesiva e inapropiada de plaquetas. Además, las SVSP activan la proteína C (no se muestra en la figura) y actúan como anticoagulantes al inactivar irreversiblemente el factor Va y el factor VIIIa. Las SVSP también pueden actuar como enzimas similares a la calicreína, convirtiendo el cininógeno en bradicinina y exacerbando las manifestaciones asociadas con los trastornos hemodinámicos.
Hipotensión
La producción de bradicinina (BK) por las serinoproteasas similares a la calicreína, así como la inhibición de la degradación de BK por los péptidos potenciadores de la bradicinina (BPP), da como resultado un aumento general en los niveles de BK. En consecuencia, la estimulación del receptor de bradicinina B2 induce vasodilatación, así como efectos antifibróticos y antiinflamatorios, a través de varios mecanismos intracelulares [149,235].
Algunas serinoproteasas aisladas, principalmente de venenos de vipéridos, pero también en elápidos como la serpiente marina Hydrophis hardwickii, muestran una actividad hipotensora similar a la calicreína que libera bradicinina y causa vasodilatación. Estas proteasas degradan la angiotensina I en angiotensina II que luego se convierte en tetrapéptidos sin actividad hipertensiva. Posteriormente, los niveles de fibrinógeno en sangre disminuyen, alterando el flujo sanguíneo, dando como resultado la liberación de BK y Met-Lys-bradicinina del cininógeno, que actúan sobre los receptores B1 y B2, causando vasodilatación y posterior hipotensión [149].
2.8. Lectinas tipo C (CTL) y similares a lectinas tipo C (CLP)
Estructura
Las lectinas de veneno de serpiente tienen propiedades intermedias entre las dos familias importantes de lectinas animales, denominadas S y C. Las lectinas de tipo C son extracelulares y tienen este nombre porque requieren iones de calcio para su actividad de interacción con azúcares. Las lectinas de tipo S deben su nombre al hecho de que requieren agentes reductores del grupo tiol para una actividad completa, tanto intra como extracelular, y muestran especificidad para los galactósidos [241].
Se han aislado y caracterizado varias lectinas de tipo C y proteínas relacionadas a partir de venenos de serpiente. Estas proteínas se pueden clasificar ampliamente en dos grupos principales según sus propiedades estructurales y funcionales: lectinas de tipo C verdaderas y un grupo más complejo de proteínas estructuralmente relacionadas con las lectinas de tipo C, pero con diferentes funciones, llamadas proteínas similares a lectinas de tipo C (CLP) [242].
Las lectinas de tipo C dependen del calcio. Son proteínas que se unen a carbohidratos con estructuras canónicas, como lo hacen las selectinas y las proteínas de unión a manosa. Son homodímeros (o multímeros superiores) con propiedades de unión a azúcares (principalmente galactósidos) (Figura 19A) [242]. Las lectinas de tipo C, del veneno de serpiente (SVgalLs), que se unen a galactósidos son un ejemplo de lectinas de tipo C verdaderas. Un ejemplo de ellas se conoce del veneno de Lachesis stenophrys (verrugo centroamericano), pero también está presente en venenos de vipéridos como Crotalus atrox (serpiente cascabel occidental) [243].
El dominio de reconocimiento de carbohidratos (CRD) en las SVgalLs está representado generalmente por tres residuos de aminoácidos, Gln-Pro-Asp, considerados determinantes en su afinidad por la galactosa, mediada por calcio. La especificidad por este carbohidrato está relacionada con la interacción de los grupos hidroxilo 3 y 4, con los residuos Q96 y D98 a través de enlaces de hidrógeno [243].
Las proteínas similares a lectinas tipo C tienen secuencias de aminoácidos similares a las lectinas de tipo C, pero no poseen propiedades de unión a carbohidratos. Además, cualquier asociación con Ca2+ no ocurre a través de la vía clásica de lectinas tipo C con el bucle de unión. Son heterodímeros, que consisten en subunidades α y β estrechamente relacionadas, generalmente unidas por un enlace disulfuro, y forman una estructura integrada mediante el intercambio de bucles. También pueden formar estructuras más grandes a través de una asociación multimérica de orden superior unida por enlaces disulfuro o por interacciones no covalentes [242]. Las proteínas similares a lectinas de tipo C (CLP) se denominan así por su alta similitud de secuencia (15%-40%) con los dominios de reconocimiento de carbohidratos de las lectinas de tipo C. Las subunidades α y β tienen pesos moleculares de 14-15 y 13-14 kDa, respectivamente. La multimerización de estos heterodímeros puede ocurrir a través de interacciones covalentes y no covalentes, lo que da como resultado estructuras αβ, (αβ)2 y (αβ)4. Los heterodímeros αβ de CLP se forman mediante intercambio de dominios, donde un dominio de una subunidad reemplaza el dominio correspondiente de la otra (Figura 19) [244].
Las CLP, también conocidos como snaclecs, presentan diversas actividades, como efectos anticoagulantes e inducción o inhibición de la agregación plaquetaria [260], según su estructura y función. La caracterización farmacológica muestra que las snaclecs pueden mejorar o inhibir la función de los factores de coagulación, lo que destaca su potencial en el descubrimiento de fármacos para enfermedades relacionadas con la sangre [244].

Figura 19. (A) Estructura de lectina de tipo C purificada a partir del veneno de serpiente Crotalus atrox. Código PDB 1MUQ. (B) Estructura de una proteína similar a lectina de tipo C llamada flavocetina-A, purificada a partir del veneno de serpiente Trimeresurus flavoviridis. Código PDB 1C3A. Moléculas preparadas en UCSF Chimera v.1.17.3
Actividades biológicas
Alteraciones hemostáticas
Las plaquetas son esenciales para la hemostasia y su agregación es el primer paso en la cascada de coagulación sanguínea (Figura 20). Este proceso depende de la interacción de muchos receptores de superficie de las plaquetas, incluidos ADP, colágeno y otros factores. Los venenos contienen componentes que alteran esta interacción, lo que provoca un sangrado continuo en la presa.
Las lectinas tipo C y las proteínas similares se dirigen a receptores específicos de glicoproteínas o integrinas para inhibir o promover la agregación plaquetaria. La mayoría de las lectinas de veneno logran sus efectos al dirigirse al receptor de glicoproteína de superficie plaquetaria GPIb, y algunas también se dirigen a GPIV, que están involucrados en la interacción colágeno-plaquetas. La convulxina, aislada del veneno de Crotalus durissus de poblaciones brasileñas, parece participar en la vía que involucra a GPIV. Las lectinas tipo C del veneno de serpiente también se unen a las integrinas, así como al factor von Willebrand (vWF), la trombina y ciertos receptores de superficie plaquetaria no identificados [246].
Los efectos de las snaclecs incluyen la actividad anticoagulante al inhibir los factores Xa y IXa y bloquear la interacción entre el factor Xa y sus cofactores en el complejo de protrombinasa que depende de Ca2+[244,247]. Por ejemplo, la inhibición de la trombina que depende del calcio [268] y la activación plaquetaria, donde las lectinas tipo C como la botrocetina y la bitiscetina de Bothrops jararaca y Bitis arietans, respectivamente, se unen preferentemente al factor de von Willebrand y agrandan la superficie de unión con el receptor plaquetario GPIb, estimulan la agregación plaquetaria [262,267]. Además, la lectina echicetina que se encuentra en el veneno de Echis carinatus tiene propiedades antitrombóticas, actuando sobre el receptor plaquetario GPIb, impidiendo su agregación [244].
Respuesta inflamatoria
Se ha investigado el papel de SVgalL en la respuesta inflamatoria utilizando diferentes modelos animales y enfoques para evaluar la participación de los leucocitos y el reconocimiento de los glicoconjugados de la matriz extracelular (ECM) en la respuesta inflamatoria. Las lectinas de Bothrops godmani (BgL) y Bothrops jararacussu (BJcuL) tienen la capacidad de inducir un aumento de la permeabilidad vascular en la pata del ratón, lo que resulta en un edema agudo moderado. Esta relación puede estar asociada con la capacidad de las lectinas para estimular la degranulación de mastocitos y liberando histamina y serotonina para desencadenar respuestas inflamatorias locales [243].
Se han observado experimentalmente otros eventos inflamatorios relacionados con las lectinas. Por ejemplo, BJcuL aumenta la adherencia de los leucocitos en los microvasos de los músculos cremáster de los ratones. Esta toxina puede unirse a las glicoproteínas fibronectina y vitronectina, lo que sugiere que la migración de los leucocitos de sangre periférica al sitio inflamatorio implica la adhesión celular a las proteínas de la matriz extracelular (ECM) mediadas por lectinas. BJcuL no solo reconoce los glicoligandos en la superficie de la célula neutrófila, sino que también promueve la polarización y la migración, mejorando la adhesión a la fibronectina y al matrigel, que son componentes de la matriz extracelular. Esta lectina induce la activación funcional de los neutrófilos al aumentar la fagocitosis y la producción de superóxido. Galatrox, una lectina del veneno de Bothrops atrox, promueve la migración aguda de neutrófilos y la liberación de las citocinas IL-1α e IL-6. Además, reconoce los glicoconjugados de la membrana celular de los neutrófilos, interactúa con la laminina de la matriz extracelular e induce la quimiotaxis de los neutrófilos in vitro [243].
Efectos sobre la función renal
En riñones aislados de ratas, las lectinas BiL, BpirL, y BmLec obtenidos de los venenos de Bothrops insulares, Bothrops pirajai y Bothrops moojeni, respectivamente, indujeron alteraciones en los parámetros de la función renal, incluyendo la presión de perfusión, la resistencia vascular, el flujo urinario, la tasa de filtración glomerular y el transporte de iones en los túbulos (Figura 20). El análisis histológico de los riñones de los animales que recibieron BiL sugiere una lesión directa de las células renales glomerulares y tubulares. El mecanismo implica citotoxicidad directa en la región glomerular inducida por las lectinas y la presencia de mediadores proinflamatorios sistémicos como las prostaglandinas [242].

Figura 20. Principales efectos de los venenos de serpientes sobre el cuerpo humano.
2.9. Péptidos y proteínas menores en los venenos de serpientes
Las técnicas ómicas (proteómica, transcriptómica y genómica), junto con los recientes avances en métodos cromatográficos y fraccionamiento de veneno bioguiado, han permitido la identificación de péptidos y proteínas que están presentes en bajas cantidades en el veneno de las serpientes. Estos componentes incluyen enzimas, proteínas sin actividad enzimática y péptidos. Las siguientes secciones describirán el rol de algunas de estas toxinas menores durante un accidente ofídico, así como sus posibles actividades biológicas.
Hialuronidasas
Las hialuronidasas son enzimas que se encuentran en el veneno de serpiente y que degradan el ácido hialurónico, el principal glicosaminoglicano de la matriz extracelular (ECM) [7,248]. Esta degradación, combinada con la escisión de otros componentes de la ECM como fibras de colágeno, laminina, nidógeno y perlecán por las SVMP, promueve la propagación de las otras toxinas en el sitio de la mordedura. Esto demuestra que una hialuronidasa de Naja naja, por ejemplo, puede aumentar la actividad miotóxica de una PLA2 y el efecto hemorrágico de una SVMP [249].
La hialuronidasa del veneno de serpiente tiene el potencial de generar fragmentos de baja masa molecular del ácido hialurónico, que pueden activar la respuesta inflamatoria del huésped [250,251]. Estos mismos fragmentos pueden activar las metaloproteasas de matriz (MMP) y aumentar el daño a la matriz extracelular (ECM). Los estudios han demostrado que estas fracciones regulan positivamente la expresión de las MMP [252].
Acetilcolinesterasas
Las acetilcolinesterasas son toxinas que hidrolizan la acetilcolina en colina y acetato. Las enzimas humanas degradan el neurotransmisor en las sinapsis, que es un mecanismo para terminar los procesos de señalización. Los venenos de elápidos suelen contener estas enzimas, excepto las mambas que contienen fasciculinas, inhibidores de las acetilcolinesterasas [253,254]. Estas enzimas podrían contribuir a la neurotoxicidad inducida por las toxinas de tres dedos y las PLA2 (como se discutió anteriormente en este capítulo) al reducir los niveles de acetilcolina en las sinapsis y promover la acción de las toxinas. Sin embargo, esta hipótesis requiere una mayor confirmación mediante investigaciones adicionales.
Nucleotidasas
Las nucleotidasas son enzimas que degradan los nucleótidos en nucleósidos. Un subgrupo de nucleotidasas son las nucleotidasas 5' que catalizan específicamente la hidrólisis del fosfato esterificado en el carbono 5' de la ribosa de los nucleótidos. Estas enzimas se han aislado de venenos de serpiente, y su principal actividad biológica es la inhibición de la agregación plaquetaria [253,254]. Por lo tanto, esta actividad puede contribuir a la inhibición plaquetaria inducida por otras toxinas, como las desintegrinas, lectinas tipo C, las PLA2 y las toxinas de tres dedos. La adenosina es probablemente uno de los nucleósidos liberados. Puede potenciar otras actividades, como la inmovilización de presas al activar los receptores neuronales de adenosina A1, suprimiendo la liberación de acetilcolina de las neuronas motoras y los neurotransmisores excitadores de los sitios centrales. La adenosina también exacerba la hipotensión inducida por veneno al activar los receptores A2 en la vasculatura. Además, la adenosina activa los receptores A3 en los mastocitos que conducen a la liberación de sustancias vasoactivas y aumentan la permeabilidad vascular [259].
Fosfolipasa B (PLB)
La fosfolipasa B hidroliza los glicerofosfolípidos en las posiciones sn-1 y sn-2 [260]. Estas enzimas se han identificado en estudios proteómicos de veneno de serpiente y análisis transcriptómicos de glándulas de veneno, incluso de especies colombianas de importancia médica como Micrurus dumerillii, Bothrocophias myersi, Porthidium lansbergii y Bothrops asper [261–264]. El primer reporte de estas enzimas en venenos de serpientes indica que presentan actividad hemolítica [265]. Las PLB posiblemente contribuyan a la lisis de los glóbulos rojos causada por otras toxinas. Sin embargo, debido a sus bajas cantidades en los venenos, no ha sido posible identificar otras actividades biológicas.
Proteínas secretoras ricas en cisteína (CRISP)
Varios venenos de serpientes contienen proteínas secretoras ricas en cisteína que se dirigen a los canales iónicos, como los canales BKCa (canales de potasio activados por calcio de gran conductancia) y los canales iónicos regulados por nucleótidos cíclicos (CNG) [266,267]. Además, las CRISP aumentan la permeabilidad vascular y las respuestas inflamatorias al activar la infiltración de leucocitos y neutrófilos [289]. Por lo tanto, las CRISP contribuyen a las actividades formadoras de edema de otras toxinas, principalmente PLA2 y SVMP.
Factores de crecimiento
Se han identificado factores de crecimiento endotelial vascular (VEGF) y factores de crecimiento nervioso (NGF) en venenos de serpiente [269,270], pero su papel en las mordeduras de serpiente aún no está completamente dilucidado. Los estudios han demostrado que los NFG y VEGF pueden inducir cambios en diferentes líneas celulares. Por ejemplo, un NGF aislado del veneno de cobra fue capaz de retrasar el crecimiento del carcinoma ascítico de Ehrlich [292]. Además, otro NGF de la cobra Naja sputatrix regula positivamente la expresión endógena de NGF en células PC12, así como los receptores de superficie celular pro-supervivencia y los canales iónicos [293]. Por otro lado, los VEGF del veneno de serpiente inducen hipotensión, permeabilidad vascular y pueden conducir a la muerte de células endoteliales [266,273].
Inhibidores de proteasa
Se han identificado varios tipos de proteasas en los venenos de serpientes, incluyendo kazal, Kunitz e inhibidores de tripéptidos; pero sus funciones en el envenenamiento no son claras aún. Los inhibidores de proteasas de tipo Kunitz tienen actividad antihemorrágica al inhibir la plasmina [274,275]. Además, se ha reportado que los inhibidores tipo Kunitz muestran actividades antiangiogénicas y antitumorales [276]. Por otro lado, un inhibidor de tipo kazal aislado del veneno de Bothriechis schelegelii no mostró efectos citotóxicos, hemorrágicos o miotóxicos. Sin embargo, indujo edema en la almohadilla de la pata de ratones e inhibió la actividad enzimática de la tripsina. Este inhibidor puede limitar la actividad proteolítica mientras el veneno se almacena en la glándula del veneno [277]. Algunos venenos de serpientes contienen inhibidores de tripéptidos que son potentes inhibidores de SVMP y que pueden atenuar, por tanto, la actividad proteolítica de las metaloproteasas en la glándula del veneno [278]. Otros componentes identificados en los venenos de serpientes incluyen fosfodiesterasas [259,279,280], aminopeptidasas [281] y vesprinas [282,283], entre otros. Sin embargo, su papel en las mordeduras de serpientes sigue siendo poco conocido.
-
Proteomas de serpientes colombianas
El veneno de las serpientes facilita su supervivencia en hábitats específicos. Comprender los venenos de las serpientes es un desafío debido a la complejidad de las toxinas, sus interrelaciones y las consecuencias fisiopatológicas del envenenamiento. Aunque las familias de serpientes venenosas comparten grupos de proteínas, cada familia tiene un grupo de proteínas dominantes que varían en su secuencia de aminoácidos y abundancia, lo que contribuye a las diferencias en la actividad biológica, tanto generales como individuales de los venenos. Por ejemplo, los venenos de vipéridos tienen una alta actividad enzimática que produce trastornos de la coagulación sanguínea, mientras que los venenos de elápidos muestran predominantemente neurotoxicidad [57].
La caracterización bioquímica de los venenos muestra que son esencialmente mezclas de proteínas y péptidos que exhiben diversas actividades biológicas [57]. La comprensión de los venenos y sus propiedades se ha vuelto más precisa con el avance de las técnicas y herramientas utilizadas para su estudio.
El análisis del veneno inicialmente implicó estudios cromatográficos agregados a la electroforesis en gel de poliacrilamida unidimensional, revelando grandes grupos de proteínas. Esto proporcionó una línea de base para estudios más específicos. Con la llegada de la cromatografía líquida asociada a las técnicas de espectrometría de masas, la electroforesis bidimensional y los métodos de secuenciación, se llevó a cabo un enfoque global que permitió visualizar la complejidad de los venenos y caracterizar individualmente las proteínas.
Existen métodos efectivos para manejar venenos crudos. Uno de ellos consiste en simplificar el veneno antes de realizar la espectrometría de masas e integrarlo con el análisis de la secuenciación por degradación de Edman [32]. Otra forma de analizar el veneno es mediante enfoques directos o de escopeta (Shotgun en inglés), utilizando cromatografía líquida acoplada a espectrometría de masas en tándem. Alternativamente, los enfoques indirectos implican analizar las fracciones cromatográficas obtenidas del veneno crudo o de las manchas resultantes de la electroforesis bidimensional (2-D).
El método de elección depende de la pregunta de investigación. El número total de proteínas únicas en el gel de electroforesis 2-D es típicamente mayor que el número determinado por el análisis directo del veneno a través de espectrometría de masas. Cada mancha puede contener más de una proteína. Esto está relacionado con la complejidad de los venenos, la presencia de múltiples isoformas y modificaciones postraduccionales de las proteínas, más que con la presencia de muchas proteínas diferentes [57].
A continuación, se propone una metodología para separar los componentes del veneno utilizando la crotamina del veneno de C. d. cumanensis como modelo (Figura 21). En el paso 1, el veneno se separa en cada componente utilizando métodos cromatográficos; luego, se selecciona una fracción y se lleva a digestión enzimática en el paso 2. Utilizando diferentes técnicas como la espectrometría de masas o la secuenciación de Edman, los péptidos resultantes se superponen para obtener la estructura primaria. El estudio de los péptidos resultantes también puede proporcionar información sobre diferentes formas (isoformas o proteoformas) de una proteína. Mediante el uso de servidores bioinformáticos especializados, la secuencia se puede utilizar para predecir un modelo tridimensional de la proteína (paso 3). El paso 4 representa una forma alternativa de identificar la secuencia seleccionando la fracción y sometiéndola a electroforesis en gel de poliacrilamida con dodecil sulfato de sodio (SDS-PAGE) para verificar su pureza. Por lo tanto, la banda se escinde del gel para ser digerida enzimáticamente (paso 5) y la identificación y modelado se realizan como se describió previamente en los pasos 2 y 3. El paso 6 destaca el uso de la fracción separada para determinar directamente la masa molecular por espectrometría de masas.
Se puede usar la herramienta de búsqueda de alineamiento local básico (BLAST) empleando las secuencias de péptidos obtenidos después de la digestión enzimática que permitieron el alineamiento con la crotamina de Crotalus durissus terrificus (poblaciones brasileras). Esta secuencia luego se utiliza para predecir la estructura tridimensional utilizando el software SWISS MODEL [228]. Estas técnicas se han utilizado para comparar venenos, determinar variaciones genéticas, ecológicas y geográficas, e incluso para construir hipótesis filogenéticas de las especies que producen toxinas específicas [57,284,285].

Figura 21. Representación esquemática de un abordaje completo de veneno, utilizando como ejemplo el veneno de Crotalus durissus (poblaciones colombianas) y su crotamina. El veneno obtenido inicialmente se separa en fracciones mediante la técnica RP-HPLC.
La cromatografía líquida en fase inversa es la técnica más utilizada para la separación de proteínas intactas. Sin embargo, no puede aislar diversas formas de proteínas (proteoformas) debido a la variación en sus propiedades fisicoquímicas. Por lo tanto, también se emplean otros métodos de separación basados en la hidrofobicidad, la interacción hidrofílica, la carga eléctrica, el tamaño y la afinidad [286]. Incluso se han descrito otras técnicas, como la electroforesis y el enfoque isoeléctrico, como posibles alternativas [287].
Sin embargo, estas técnicas de separación pueden no siempre resolver eficazmente proteomas complejos. Por el contrario, las estrategias proteómicas de «arriba hacia abajo» (Top-down procedure en inglés) se pueden utilizar para identificar y caracterizar proteínas fragmentando proteínas intactas, en lugar de medir los péptidos producidos a partir de proteínas mediante procedimientos de digestión tríptica [198]. El análisis directo de proteínas intactas proporciona datos enriquecidos, que cubren casi la secuencia completa de proteínas, y permite la identificación de proteoformas y la localización de modificaciones [288]. Actualmente, esta técnica está limitada a proteínas de tamaño bajo y mediano. Sin embargo, probablemente se utilizará para complejos proteicos más grandes en el futuro cercano.
Los proteomas de venenos de serpiente se representan típicamente de manera gráfica como diagramas circulares o de barras, que muestran el porcentaje de abundancia de cada familia de proteínas y su relación con la actividad general del veneno (ver Capítulo 3). En Colombia, el veneno de los vipéridos (Figura 22), muestra una mayor concentración de metaloproteasas en especímenes juveniles de Bothrops asper que tiende a disminuir en individuos adultos. Esta diferencia se compensa con un ligero aumento en la presencia de PLA2, serinoproteasas y L-aminoácido oxidasas [289]. En el veneno de Bothrops atrox, las metaloproteasas representan casi el 50% del proteoma. Si bien PLA2 también está presente, vale la pena señalar que el veneno de las poblaciones colombianas contiene dos veces la cantidad de serinoproteasas y L-aminoácido oxidasas reportado para las poblaciones brasileñas [290].
El veneno de Porthidium lansbergii mantiene la relación 2:1 entre metaloproteasas y PLA2, pero también contiene una cantidad significativa de desintegrinas y lectinas tipo C (13% y 7%, respectivamente) [291]. Aunque el veneno de P. lansbergii es rico en estos componentes, los estudios de sus aplicaciones biotecnológicas aún están en sus primeras etapas [292]. En contraste, el veneno de Bothrocophias myersi (Cabeza de sapo roja) está compuesto principalmente de PLA2 y SVMPs (54% y 21,4%, respectivamente) [284]. Los venenos de otras víboras, como Bothrocophias campbelli (cabeza de sapo chocoana) contienen menos del 1% de desintegrinas [198], y el veneno de Crotalus durissus tiene lectinas tipo C que representan el 1,18% de su proteoma [293].
En particular, el veneno de C. durissus está compuesto por más de un 60% de crotoxina, una PLA2 neurotóxica, y un 6% de crotamina, una miotoxina de bajo peso molecular que es típica de las serpientes de cascabel [293] (Figura 22). En contraste, Lachesis acrochorda (verrugoso chocoano) tiene un predominio de serinoproteasas, seguidas de metaloproteasas y péptidos vasoactivos, incluyendo toxinas poco comunes como los péptidos potenciadores de bradicinina (BPP) y el péptido natriurético tipo C (C-NP) [294].

Figura 22. Representatividad de toxinas en los proteomas de venenos de víboras colombianas con datos disponibles. BPPs: péptidos potenciadores de bradicinina; PLA2: fosfolipasa A2; SVSP: serinoproteasa de veneno de serpiente; SVMP: metaloproteasa de veneno de serpiente; LAAO: L-aminoácido oxidasa; CRISP: proteína secretora rica en cisteína; CTL: lectinas tipo C y similares a lectinas; DIS: desintegrina; NGF: factor de crecimiento nervioso; VEGF: factor de crecimiento endotelial vascular; PEP: otros péptidos; UNK: desconocidas; Crotoxina: neurotoxina cristalina de tipo fosfolipasa A2 obtenida del veneno de la serpiente de cascabel del género Crotalus; Crotamina: miotoxina de bajo peso molecular del veneno de la serpiente de cascabel del género Crotalus. Los proteomas del veneno de las especies se obtuvieron de [81,290,293,295–297].
Solo ocho especies de elápidos colombianos tienen caracterizaciones proteómicas disponibles. Al considerar la dicotomía de 3FTx-PLA2, en el veneno de Micrurus mipartitus, las 3FTx predominan con 61% [285], mientras que para M. dumerilii,M. helleri,M. medemi y M. sangilensis el 52%, 41%, 43% y 30% del proteoma está representado por PLA2, respectivamente [264], que incluye proporciones significativas de péptidos de Kunitz para M. dumerilii y metaloproteasas para M. helleri, M. medemi y M. sangilensis (Figura 23). El veneno de la serpiente marina de vientre amarillo Hydrophis platurus de la población costarricense contiene aproximadamente 50% de 3FTx, en comparación con 33% de PLA2 [297], sin embargo, aún no se ha caracterizado ninguna población colombiana.

Figura 23. Representatividad de toxinas en los proteomas de venenos de serpientes corales colombianas con datos disponibles. PLA2: fosfolipasa A2; SVSP: serinoproteasas de veneno de serpiente; SVMP: metaloproteasas de veneno de serpiente; LAAO: L-aminoácido oxidasa; CRISP: proteína secretora rica en cisteína; CTL: lectinas tipo C y similares a lectinas; 3FTx: toxinas de tres dedos; KUN: péptidos de Kunitz. Los proteomas del veneno de las especies se obtuvieron de [264,285].
Aunque se ha incrementado la caracterización de los proteomas de veneno de serpientes de especies colombianas, son necesarios más estudios. Debido a las bajas tasas de encuentro, hábitats crípticos y alta riqueza de especies, la mayoría de los venenos de las serpientes de importancia médica en Colombia carecen de caracterización proteómica de sus venenos, siendo las corales (Micrurus) las que presentan los mayores vacíos de conocimiento. Conocer las principales toxinas del veneno de cada especie permitirá entender las actividades fisiopatológicas de cada componente y sus potenciales aplicaciones en biotecnología y medicina.
Potenciales usos de los venenos de serpientes
Los venenos de serpientes son mezclas complejas que contienen toxinas que atacan varios procesos fisiológicos. Han sido reconocidos como fuentes de moléculas bioactivas desde el descubrimiento y desarrollo del captopril (ver Capítulo 10). Ha habido casos exitosos en los que se han producido medicamentos a partir de toxinas de serpientes. Por ejemplo, el tirofibán es un medicamento utilizado para inhibir la agregación plaquetaria en síndromes coronarios agudos. La desintegrina identificada en el veneno de Echis carinatus fue modificada y formulada para desarrollar este medicamento [298].
Los venenos de serpientes también son útiles para estudiar procesos biológicos. Por ejemplo, la batroxobina se utiliza con fines diagnósticos en química clínica. La reptilasa (Reptilase®, Pentapharm, Basilea, Suiza) es una alternativa al tiempo de trombina en muestras que contienen heparina o para diagnosticar hipofibrinogenemia [299]. Los venenos son fuentes importantes de compuestos bioactivos que tienen potencial como compuestos principales para el desarrollo de fármacos o como moléculas para estudiar algunos procesos biológicos (ver Capítulo 10).
Apéndice: Materiales y métodos
Visualización de estructura secundaria de toxinas en 3D
En este capítulo el estudio de las toxinas se aborda desde la estructura secundaria. Para su correcta visualización se colorean selectivamente las zonas con estructura secundaria en hélice alfa (en fucsia), hoja beta (en verde/amarillo), giros y estructura al azar (en blanco).
Se escogió el modo de visualización esquemático para permitir mostrar la representación de Richardson (MolScript). Esta visualización es similar al modelo de cintas, pero muestra mediante puntas de flecha la orientación de las cadenas en hebras y hélices, y los tramos sin estructura secundaria son cordones en lugar de cintas [301,302]. En algunas proteínas, o en ciertas regiones de estas, no existen interacciones de suficiente consideración como para que se pueda distinguir un nivel de organización superior a la estructura primaria. En estos casos se habla de conformación al azar.
Cuando la cadena principal o esqueleto de un polipéptido se pliega en el espacio en forma de helicoide dextrógiro se adopta una conformación denominada hélice alfa. Por lo contrario, cuando la cadena principal de un polipéptido se estira al máximo permitiendo que sus enlaces covalentes interaccionen, este adopta una configuración espacial denominada estructura b (hoja beta), que suele representarse como una flecha [302]. Cuando las estructuras beta tienen el mismo sentido, la hoja b resultante es paralela, y si las estructuras b tienen sentidos opuestos, la hoja plegada resultante es antiparalela.
Las secuencias de la cadena polipeptídica con estructura alfa o beta a menudo están conectadas entre sí por medio de los llamados giros beta, que son secuencias cortas, con una conformación característica que impone un brusco giro de 180 grados a la cadena principal de un polipéptido. Los modelos 3D de las toxinas fueron obtenidos de la base de datos Protein Data Bank (PDB), fueron descargados en formato Wavefront(.obj) y posteriormente optimizados utilizando Blender [303,304]. Posteriormente fueron importados a Jmol generando el patrón de estilo esquemático, luego se exportaron en formato 3D VRML [305]. Finalmente se importaron a la plataforma sketchfab para generar el código html de visualización [306].
